Bleaching powder manufacturing process, uses, reactions
Bleacing powder is calcium hypochlorite ( Ca(OCl)2 ). It is a one of the major chemical industry in the world. Limestone and chlorine gas are used as raw materials to manufacture bleaching powder which is used as a disinfectant and as an oxidizing agent. Bleaching powder show different reactions.
In this tutorial, we study about followings.
- What is bleaching powder?
- Manufacturing process
- Uses of bleaching powder
- Reactions of bleaching powder
What is bleaching powder
Bleaching powder is a white - yellowish powder which very well dissolves in water. The active ingredient of bleaching powder is calcium hypochlorite ( Ca(OCl)2 ). It is an inorganic compound.
Bleaching powder composition
Bleaching Powder chemical composition: Ca(ClO)2CaCl2Ca(OH)2.2H2O
Calcium hypochlorite - Ca(OCl)2
Ca(OCl)2 is a white, corrosive solid that comes either in tablet form or as a granular powder in the market. It easily emits chlorine gas easily when it contacts with water or moisture.
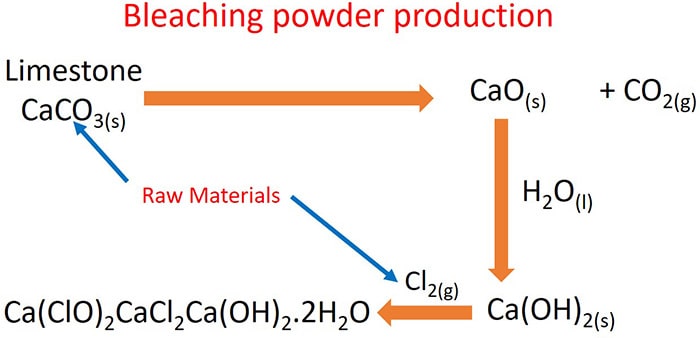
Raw Materials of bleaching powder manufacturing process
- Limestone (CaCO3) - to obtain calcium oxide
- Chlorine gas (Cl2)
Bleaching powder manufacturing process
Steps, reactions, materials of bleaching powder and physical conditionsin manufacturing process is explained below.
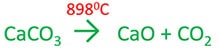
Heating limestone
First, slaked lime (CaO) is produced by heating limestine. As a by product carbon dioxide is given.
Calcium oxide and water reaction
Calcium oxide is mixed with water to take calcium hydroxide ( Ca(OH)2 ). Ca(OH)2 is precipitated in concentrated solutions.
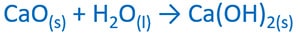
Calcium hydroxide and chlorine gas rection

Bleaching powder manufacturing process in industrial plant
Bleaching process of bleaching powder
Reason to bleaching property is hypochlorite ion (OCl-). Colourful things are oxidized by atomic oxygen ('O') which is formed by OCl- ion. Atomic oxygen is very reactive.
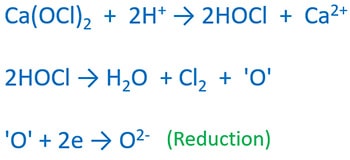
when atomic oxygen is reduced, Colorful things are oxidized simultaneously.
Fe2+, I-, Br- ions can be oxidized by bleaching powder.
Uses of bleaching powder
- As a bleach.
- As a disinfectant. As an example, in water treatement processes.
- To manufacture chloroform
Explain uses of bleaching powder in detail
Purifying water using bleaching powder in purification plants
A suspension of bleaching powder in water is added dropwise to water and stirred. In earlier times, chlorine gas (Cl2) was used. But due to inconvenience of transporting heavy gas cylinders of chlorine, this method is abandoned.
Reactions of bleaching powder
Dilute acids and bleaching powder reaction
When bleaching powder is heated with a dilute acid, chlorine gas is emitted.
Reaction of air and bleaching powder
Gaseous CO2 dissolve well in the water. That forms carbonic (H2CO2) acid. Carbonic acid can release Cl2 from bleaching powder.
Bleaching powder and water reaction
Bleaching powder hydrolysis to hypochlorous acid ( HOCl ) and calcium hydroxide ( Ca(OH)2 ). Hypochlorous acid is not stable and again hydrolysis to chlorine gas.
Oxidation characteristic of bleaching powder
Bleaching powder can oxidize another substances. Therefore bleaching powder can behave as an oxidizing agent.
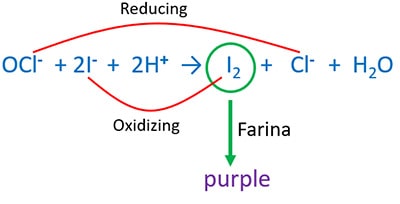
Acidic KI and bleaching powder reaction
Add bleaching powder to acidic KI solution. Purple colour I2 is emitted. When I2 contact with farina, you can see purple colour.
Acidic Fe2+ solution and bleaching powder reaction
Ferrous ions ( Fe2+ ) are oxidized to ferric ( Fe3+ ) ions. You can identify Fe3+ ions by adding KSCN. Then red colour [Fe(SCN)]2+ is given.

What is the commercial bleaching powder mixture?
Ca(ClO)2CaCl2Ca(OH)2.2H2O
Does ammonia react with bleaching powder?
Bleaching powder reacts with ammonia and give calcium chloride ( CaCl2 ), nitrogen gas (N2 ) and water as the products.
What are the other disinfectants can be used insted of Ca(OCl)2
Sodium hypochlorite (NaClO) can be used as a disinfectant. But HOCl is more efficient than NaClO and Ca(OCl)2 in disinfecting process.
Bleaching process of HOCl
HOCl decomposes to HCl, atomic oxygen atom and water. Atomic oxygen is very reactive and is reduced to O2- readily. With that step, coloured thing is oxidized to get colourless.
Related Tutorials
Environmental Pollution due to Industrial Activities