Calcium carbide and acetylene manufacturing process
Calcium carbide is a very useful chemical in industrial applications and domestic uses. We can prepare acetylene gas by the production of calcium carbide. In this lesson we discuss about preparation of calcium carbide and acetylene gas and then their uses in chemical industry and domestic purposes.
Calcium carbide and acetylene gas
Brief description of calcium carbode other compounds are given below.
- Calcium carbide - CaC2
- Acetylene gas (Ethyne) - C2H2: This is a combustible gas.
Preparation of calcium carbide
Raw materials
- Limestone / Calcium carbonate (CaCO3)
- Coke (C)
- Water is required to production of acetylene gas
Production Process
Steps of production, reactions and physical conditions are discussed in this section.
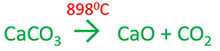
Calcium carbonate decomposition | CaCO3 = CaO + CO2
Calcium carbonate is heated into higher temperature and take calcium oxide (CaO). CaCO3 decomposes into
CaO and CO2 above 8250C.
Calcium oxide and coke reaction | CaO + C = CaC2 + CO
Produced CaO and coke are heated at temperature around 20000C in an electric arc furnace. Calcium carbide and
carbon monoxide are produced in this step.

Preparation of acetylene from calcium carbide
Calcium carbide and water reaction | CaC2 + H2O = HCCH + Ca(OH)2
Add calcium carbide into water. You can see a smoke is coming out of water. This smoke is the acetylene gas which is highly flammable. Acetylene's IUPAC name is ethyne. Which is an organic compound and belongs to alkyne group.
Acetylene gas is an organic compound and belongs to alkyne group.
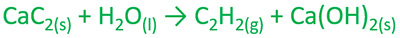
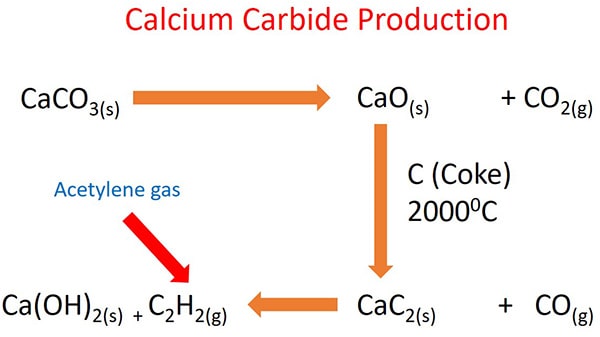
Calcium carbide production process
Following figure shows, how CaC2 and acetylene gas is manufactured by starting from raw materials and what are the physical conditions you should maintain to get higher yield.
Carbide compounds can be prepared from strontium and barium metals too.
Uses of acetylene gas
- to produce oxyacetylene flame for welding industry.
- Produce polymers
- to ripen fruits
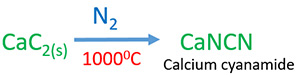
Preparing calcium cyanamide
Calcium cyanamide(CaNCN) is a fertilizer. Calcium carbide is heated with nitrogen gas stream in 10000C to manufacture calcium cyanamide.
Problems of calcium carbide manufacturing process
Calcium carbide plant causes to environmental pollution.
- Corol and limestone are used to take CaO. This causes to coastal erosion.
- Energy required for preparing CaC2 is higher than getting energy by the combustion of C2H2.
- Generated heat causes to increase the temperature of environment.
- Cannot transport ethyne (C2H2) easily.
- Storing ethyne is difficult.
Calcium carbide in rippening foods
Calcium carbide is a prohibitted compound to rippen foods in most countries.
What is the colour of calcium carbide?
Pure calcium carbide is colourless. But pieces of technical-grade calcium carbide are grey or brown colour because existing of calcium oxide, calcium phosphate, calcium sulfide, calcium nitride and silicon carbide.
Calcium carbide to ethyne
Ethyne is the IUPAC name of acetylene. Add water to calcium carbide. It gives ethyne gas which has two carbon atoms and two hydrogen atoms.
What are the other methods of preparation of acetylene?
- Heating 1,2-dibromoethane with alcoholic strong alkali such as NaOH or KOH.
- Heating suitable ethane-1,2-diol with dehydrators such as concentrated H2SO4, Al2O3 or P2O5.
Related Tutorials to Calcium Carbide and Acetylene Gas