Industrial Nitric acid (HNO3) Manufacturing Process, Raw Materials, Uses, Ostwald Process
Nitric acid is a very common and heavily used chemical in the world and manufactured in large scale by global manufactures. Nitric acid manufacturing process is started from ammonia gas (NH3) and Oxygen gas (O2) in the Ostwald process. Potassium nitrate and sulfuric acid also can be used to produce Nitric gas in laboratory scale. Nitric acid is used to manufacture explosives, some metal nitrates and there are many more applications. In this tutorial, we will look all aspects of Nitric acid manufacturing from beginning to end product.
Content
- Brief summary about characteristics of nitric acid
- Prepare nitric acid in laboratory
- Industrially manufacturing of Nitric acid from Ostwald method
- Reactions of nitric acid
Brief summary about characteristics of nitric acid
Characteristics of Nitric acid should be thoroughly examined because those characteristics affect the production process, safety, storage facilities during the production process.
- A strong acid: Aqueous HNO3 acid is a strong acidic solution and reacts with metals.
- A strong oxidizing agent: As aqueous HNO3 become a strong acid, it is strong oxidizing agent because Nitric acid can be
reduced to lower oxidation states
such as Nitric oxide (NO) or
Nitrogen dioxide (NO2) while other chemical is
being oxidized.
- Decomposes when exposes to the sunlight: Nitric acid decomposes to Nitrogen dioxide, oxygen and water when it exposed to sunlight or
higher temperatures.
Prepare nitric acid in the laboratory in small scale requirements
Nitric acid can be produced in an analytical laboratory in small scale under safety conditions under experienced technical supervisor. Use fume hood to eliminate any toxic gases formed duration the procedure and wear all personnel protective equipments (PPEs).
Reaction of Potassium nitrate and concentrated sulfuric acid
The reaction of potassium nitrate and concentrated sulfuric acid can be used to prepare nitric acid in the laboratory. When mixture is heated, formed nitric acid can be separated by distillation process.

Industrially manufacturing of Nitric acid from Ostwald method
Here, we will start to looking the every aspects of Nitric acid manufacturing about raw materials, process conditions, health and safety, waste generation, and many more.
Raw materials used in industrial nitric Acid manufacturing process
There are three main raw materials in Nitric acid manufacturing Ammonia gas, Oxygen gas and water.
Ammonia gas (NH3) as the source of nitrogen
Ammonia is used as the source of Nitrogen for Nitric acid manufacturing. Ammonia gas is industrially manufactured by Haber Process. Ammonia is fed to the reactor as a gas for further reactions. Storage and handling of ammonia gas should be handled with care.
Oxygen gas (O2) to oxidize ammonia and Nitric oxide
Oxygen gas is used to oxidize ammonia to Nitric oxide and Nitrogen dioxide prior to Nitric acid production. Purified Oxygen gas can be separated from ambient air.
Water to produce Nitric acid
Water is used in the last stage of Nitric acid manufacturing when Nitrogen dioxide gas is dissolved in water. Though, water is not used as a raw material, it can be used as coolant where it necessary.
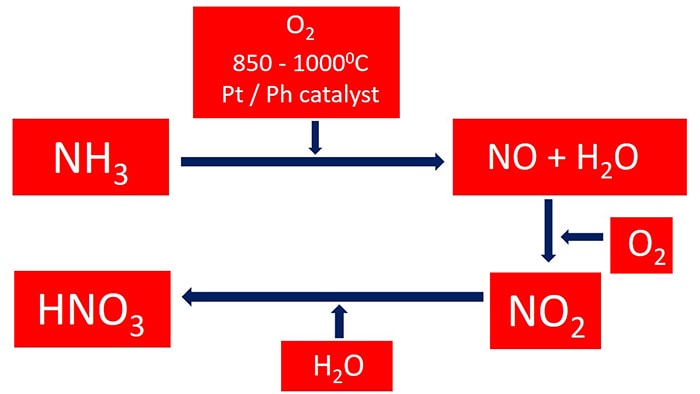
Nitric acid manufacturing process - Ostwald method
Step 1
Prepare nitric oxide (NO)
- Ammonia (NH3) gas and Oxygen (O2) gas are mixed to 1:9 volume ratio.
- The mixture is heated up to 850 - 10000C temperature. That mixture send through Pt/Rh catalyst. Then exothermic reactions happens and produces NO and H2O as results. Due to exothermic reaction (heat is generated as a result of reaction), reaction happens easily after it was started.
4NH3(g) + 5O2(g) → 4NO + 6H2O(g) + ΔH
ΔH0 = -904 kJmol-1
- Reaction happens under high pressure conditions. To create a higher pressure, more reactants has to be entered to the reaction. This will increase more impacts between reactants and catalysts. This increases reaction rate to produce more products.
- Excess air will confirm completeness of ammonia oxidization to Nitric oxide (NO).
Step 2
Prepare Nitrogen dioxide (NO2)
- Next reaction give us Nitrogen dioxide (NO2(g)) as the result of furthermore oxidation of Nitric oxide. This reaction should be done at low temperature than previous reaction. (<1500C). Hence produced Nitric oxide is cooled and cold air mixed with Nitric oxide. NO reacts with excess O2
NO(g) + O2(g) → NO2(g) + ΔH
ΔH0 = -115 kJmol-1
Step 3
Prepare nitric acid as the final product
- Produced Nitrogen dioxide (NO2(g)) in the second step is now cooled before it sends to the water.
- Nitrogen dioxide is absorbed very well in the water and produce Nitric acid as a result of furthermore oxidization.
3NO2(g) + H2O2(l) → 2HNO3(aq) + 4NO(g)
Released nitric oxide (NO) in step 3 is resent back to the step 2 to oxidize to Nitric oxide.
Requirements for 96% translation in industrial nitric acid manufacturing
- Pressure: 4-10 atm
- Temperature: 850- 10000C
- Pt catalyst (10% Rh content)
Uses and applications of nitric acid in industries
- To produce explosives such as TNT, TNG
- Important chemical
- Get dissolved metals
- To produce fertilizers
- To produce metal nitrates:
- AgNO3: photographic films and paper
- KNO3: Gun powder
- NaNO3: preservation for meat, manufacture of sausages
Environmental pollution due to nitric acid manufacturing industry
As other chemical industries in the world, nitric acid production causes to different environmental pollution cases.
- Water is used to reduce temperature of the system. When that water is released to the environment there may be effects to animals and plants. Therefore, recycling water to the manufacturing system is recommended to minimize the effect.
- Nitric oxide, Nitrogen dioxide gases cause to acid rains, damages to Ozone layer, photochemical smog.
Health and safety aspects in Nitric acid manufacturing plant
There are hazardous in an industrial area and we are going to identify what are the them in a Nitric acid manufacturing plant.
- Storage of ammonia gas
- High pressure vessels
Storage of ammonia gas
Ammonia gas is a highly toxic gases to humans and exposure to small quantities us enough to deaths. Also, ammonia is a combustible chemical. Avoid storage of bulk quantities of ammonia inside the production plant.
It is recommended that installing ammonia gas sensors and warning system to identify any leakages. Working team should wear all respiratory protection wearings within manufacturing area.
High pressure vessels
Because reactions are taken place at high pressure and high thermal conditions, sudden explosions are another hazard in this industry. Continuous monitoring pressure conditions, setting automatically cut-off are few solutions for this kind of hazards.
Have Questions?
What are the raw materials required for the manufacture of nitric acid by Ostwald process
Ammonia, oxygen, water are the raw materials of nitric acid manufacturing. Ammonia can be obtained by haber process. Ammonia gas is oxidized to nitrogen dioxide by oxygen. Nitrogen dioxide is dissolved in water.
Name the commercial process to used to prepare nitric acid
Ostwald process is the commercial process to used to prepare nitric acid in industrial scale.
What are the nitric acid production methods
- Potassium nitrate reacts with concentrated nitric acid on heating to give nitric acid. Nitric acid is separated by distillation. This method can be used in laboratory.
- Using ammonia and oxygen, nitric acid can be prepared. This is the industrial method, Ostwald process.
What are the catalyst used in large scale preparation of nitric acid?
In ammonia oxidation to NO, Pt is used as the catalyst.