Sulfuric Acid (H2SO4) Manufacturing Process | Contact Process
Sulfuric acid is one of the most important chemical produced in the world. Sulfuric is used in other chemical industries and have many uses in laboratories too as a chemical compound. In this tutorial, we are going to learn about followings.
- Brief introduction to sulfuric acid and its characteristics
- Sulfuric acid manufacturing processes: raw materials, process parameters such as temperature, pressure
- Uses of sulfuric acid
- Environmental pollution due to sulfuric acid production
Sulfuric acid (H2SO4) characteristics
Sulfuric acid is a strong dibasic acid.
Releasing sulfuric acid to the environment may cause many harmful effects to humans, animals and also to the environment.
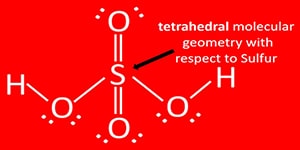
Oxidation number of sulfur atom in the sulfuric molecule is +6. Molecular shape of sulfuric molecule is tetrahedral.
Sulfuric acid manufacturing processes
There are two ways to produce sulfuric acid in industry.
- Lead Chamber process
- Contact process
In this tutorial, contact process is discussed in detail.
Contact process of sulfuric acid manufacturing
Contact process is the most widely used method of sulfuric acid manufacturing. In this method, Sulfur dioxide gas is produced by combustion of different sulfur containing materials.
Raw materials for sulfuric acid manufacturing process in contact process
- Sulfur of sulfide compound - to produce SO2 gas
- Air - to burn sulfur or sulfur compounds
- Pre manufactured sulfuric acid - to dissolve produced sulfuric acid
- Water
Taking sulfur as a by-product from petroleum industry
Crude oil contains sulfur as a minor element. There are very strict environmental guidelines for sulfur content in the diesel and petrol, therefore sulfur is reduced to very low level in petroleum products.
Therefore, sulfur is formed in the desulfurisation process of petroleum. This removed sulfur is available at very low cost. Therefore, many sulfuric acid manufacturing countries use that sulfur in their sulfur dioxide preparing process.
Process of catalysts in contact process
- Platinum (Pt) or Palladium (Pd) or vanadium pentoxide (V2O5) can be used as catalysts.
- Platinum is more efficient. But Platinum get poisoned rapidly in the presence of sulfur.
- Hence we have to use vanadium pentoxide. But, vanadium pentoxide has low efficiency. But, because it is a cheap material, it is an advantage to use it in the process.
Sulfuric acid manufacturing process
Sulfuric acid manufacturing is a long process and catalysts, heating, high pressures, Le chatelier's principle are applied during the complete process.
Step 1
Burning sulfur or sulfide compound
Sulfur or sulfide compound is burnt in air to produce sulfur dioxide gas (SO2). For sulfide compounds, ZnS, PbS, FeS can be used.
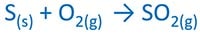
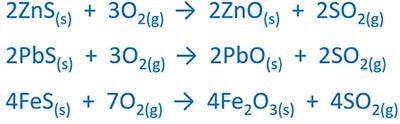
Step 2
Conversion of sulfur dioxide to sulfur trioxide | SO2 to SO3
Furthermore sulfur dioxide is oxidized to
sulfur trioxide (SO3) by supplying
oxygen gas. This reaction is a reversible one.

Activation energy of this reaction is very large. Thus reaction does not happens spontaneously . Catalyst are applied in splendid high temperature. SO2 is passed through several catalyst beds to convert SO3. Then, Sulfur trioxide is removed from the last catalyst bed. It will shift the equilibrium towards right side of the reaction. This reaction is exothermic and rate of the reaction is small when catalyst is not exist.
To accelerate the reaction rate, several approaches are possible.
- Use of a catalyst
- Increase of temperature
- Use of excess oxygen
- Increase of pressure
Effect of temperature
In low temperatures, reaction rate is slow. But in higher temperatures , the reaction slows down because this reaction is exothermic. Hence we use about 4500C. This is the same situation we faced in haber process when manufacturing ammonia.
Effect of pressure
In forward reaction, number of molecules are decreased. Hence pressure is reduced. According to the le chatelier's principle, applying high pressure to this system will give successful product. But above conditions we have a good product rate and amount, we do not need extra pressure. Cost is high when apply high pressures because affordable new equipments are required. Finally 1-3 atm pressure is good for converting SO2 to SO3.
Excess O2 will increase forward reaction rate.
Catalysts used in sulfur dioxide to sulfur trioxide
Platinum or vanadium oxide can be used as catalyst. Both have advantages and drawbacks.
advantages and drawbacks of platinum and vanadium hydroxide as catalysts
- Platinum (Pt) is more efficient than vanadium pentoxide (V2O5). But in the presence of sulfur platinum gets easily poisoned. But vanadium pentoxide does not get poisoned easily.
- Pt is also very expensive compared to vanadium pentoxide.
sulfur dioxide is past through several catalyst beds.
Step 3
Reaction of sulfur trioxide and water
Reaction of sulfur trioxide and water is very passive one. Reason is, water vapor
from the system reacts with SO3 easily and forms H2SO4 smog.
That smog does not dissolve in water easily.
Therefore, SO3 is dissolved in a 98% - 99% pre manufactured sulfuric acid solution. Sulfur trioxide is more
soluble in sulfuric acid and form fuming sulfuric acid.
Fuming sulfuric acid - H2S2O7
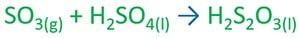
Fuming sulfuric acid is diluted with water to produce sulfuric acid
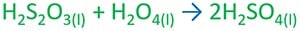
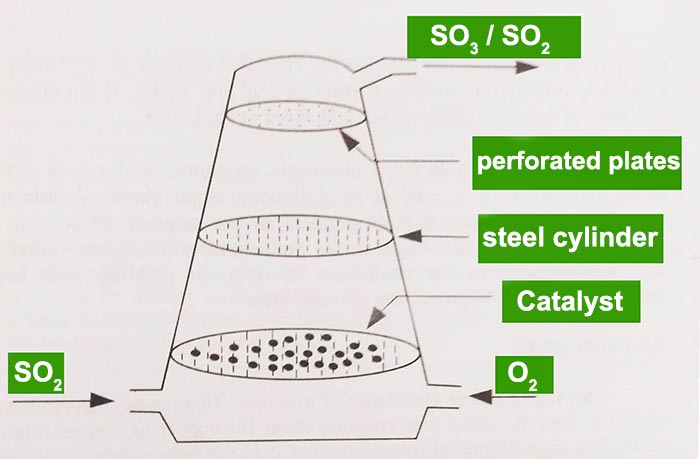
Conditions used in contact process
- vanadium pentoxide catalyst
- a temperature of 5000C
- excess air
- 1 atm pressure
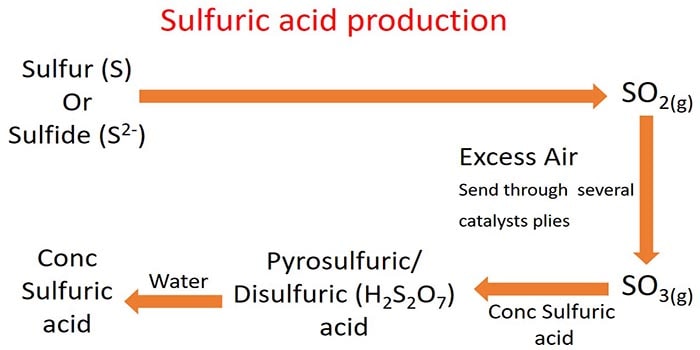
Figure 02: Sulfuric acid manufacturing process
why is the contact process important?
Uses of sulfuric acid
- Produce superphosphate fertilizer from apatite.
- produce colorings
- Produce HCl in the laboratory
- to fill motor vehicle batteries
Environmental pollution due to sulfuric acid production
As other chemical industy, there is an environmental pollution in H2SO4 production.
In sulfuric acid production, sulfur dioxide and sulfur trioxide gases are formed. These gases are acidic. If they are leaked to the air, they will form sulfuric acid and cause to acid rains.
If sulfuric acid is leaked into a water stream, natural water becomes acidic and cause so many health problems.
450 - 500 0C temperature is used in the plant. Large heat will increase the temperature of environment.
Improving efficiency of the contact process
As every other chemical indstry, sulfuric acid manufacturing plant try to maximize its production by using less raw materials and less energy to take more profit. In this section, we try to look, what actions we can take to increase the efficency.
Pre heat from heated materials
In sulfur trioxide producing step, around 4500C temperature should be maintained. In the combution process of sulfur dioxide producing, a large heat is generated. We can use that heat for the second step of the process. That will reduce eneergy requirement of the plant.
- Standard enthlpy of combution of sulfur = -296.8 kJ/mol
Anhydrous calcium sulfate with coke to produce sulfur dioxide
This is another method to produce sulfur dioxode gas.
- First, calcium sulfate is heated with coke to give calcium sulfide and carbon dioxide.
- Next, produced calcium sulfide reacts with calcium sulfate to give calcium oxide and sulfur dioxide.
Questions
What catalyst is used in contact process?
Platinum (Pt) or vanadium oxide (V2O5) is used as catalyst.
Give contact process conditions to get better yield
V2O5 is used as the catalyst and around 5000C temperature is applied with 1 atm presssure.
What is the oxidation number of H2SO4
We know sulfur can show variable oxidation numbers from -2 to +6. Also electronegativity of hydrogen is less than other two elements, sulfur and oxygen. Therefore, hydrogen has +1 oxidation number. Oxygen is the most electronegative element and usually has the -2 oxidation state.
Finding oxidation number
take oxidation number of sulfur as x.
- sum of oxidation numbers of all elements = 0
- +1*2 + x + (-2)*4 = 0
- x = +6
Can we make sulfuric acid from petroleum industry?
Yes. Petroleum industry is a good source for sulfur to make sulfuric acid. It makes hydrogen sulfide gas at unifying stages in gasoline and diesel production.
Then, what is the problem?
Yes, hydrogen sulfide is prepared. But not only H2S. There are other impurities with H2S. So we have to separate hydrogen sulfide from the mixture and feed it to excess oxygen gas for combution. It gives SO2.
in contact process, what is the the gas produced?
As gases, sulfur dioxide (SO2) and sulfur trioxide (SO3) gases are produced.