Urea is a very important industrial production which is much used in agricultural field as a fertilizer because urea contains high percentage of nitrogen. Urea dissolves very well in water. Urea is called also as carbamide, which is an organic compound with chemical formula of CO(NH2)2. Urea is a white solid compound.
Urea is an amide compound and has two -NH2 groups connecting to the carbonyl group.
In this tutorial, we first discuss urea manufacturing process, raw materials, reactions and uses of urea. Then study some useful reactions of urea and urea manufacturing plants in the world.
Because urea production is a industrial thing, there are so many things to discuss about manufacturing process of urea. In this section, we will discuss about raw materials, process conditions, environmental pollution due to urea industry.
Raw materials are the things which are used to manufacture urea. These raw materials are taken from other industries or produced themselves inside the plant.
Ammonia is manufactured by haber process in the industry.
Carbon dioxide (CO2) is prepared by decomposition of limestone (CaCO3). When CaCO3 is heated, it decomposes to CaO and CO2. (When alkali earth metal carbonate decomposes, it prouce carbon dioxide and metal oxide).
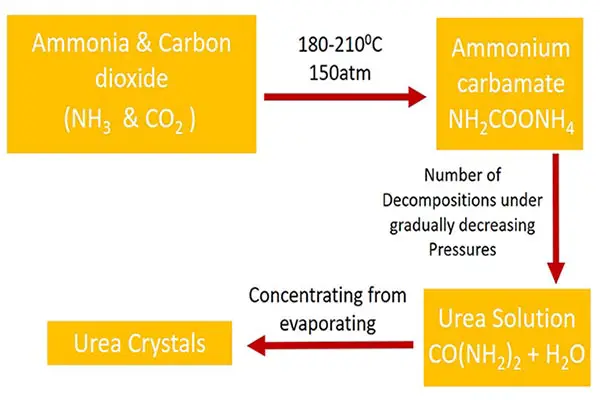
Liquid ammonia is allowed to react with liquid carbon dioxide in a reactor at high temperature and pressure. The conditions employed are 130-1500C and a pressure of 35 atm. urea is formed in two-step reactions.
First step,
Ammonia and carbon dioxide react together and give ammonium carbamate (NH2COONH4).

Fast, Exothermic , Go to completeness at industrial situations.
Second Reaction,

Slow, Endothermic , does not go to completeness.
Manufactured urea contains unreacted ammonia and carbon dioxide and ammonium carbamate. Ammonium carbamate is removed by reducing the pressure (Le Chatelier's Principle). When heating, ammonia and carbon dioxide is separated from the product mixture. The advantage of this process is ammonia and carbon dioxide can be recycled back to the process. That will reduce the cost of raw material.
Urea is obtained as a solution, but that solution is concentrated to give 99.6% molten urea, and granulated for use for fertilizer.
For the production, heat should be supplied which cost large money. If urea production company can reduce energy requirement by saving energy, company can gain more profit.
Urea production may cause several environmental pollution problems. These problems occur due to poor maintenance of the plant, leakages of toxic materials to the natural environment and more.
KOCH Fertilizers is a leading urea manufacturing company in USA. They have other fertilizers too. They produce 13 million tons of different fertilizer products annually.
According to the stoichiometry of urea production reactions, two moles are required to produce 1 mol of urea.
India is the top country of urea production. China, Indonesia, Russia, Qatar are the next other countries of urea production.
Ammonia is the most toxic material used in the urea manufacturing. Carbon dioxide is not much dangerous when it compares with ammonia. But it causes to increase the temperature of the environment.
Urea manufacturing second reaction is not going to completeness. So to get maximum output, we have to maintain the temperature and pressure properly. Otherwise prouction quality decreases due to low production.
Urea cannot be produced by a single reaction. It requires two steps reactions to get the product. First, ammonium carbamate is produced and next ammonium carbomate decomposes to urea and water.
It is not possible to manufacture urea in home as a chemical because it requires well designed plant to work. Also it requires very high temperature which is impossible to get in houses. In addition, the safety should be considered in higher place due to hazardous chemicals.
Every industrial plant, different equipments are worked. Sometimes those machines generate lot of heat. So we need to remove that heat to protect those machines by high temperatures. So we use water as a coolant for this purpose.
Nitrogen content of urea is very high. Usually it may around 46%. And urea's water solubility is high. Due to that reason absorbing urea to plants is easy.