Transition metal compounds oxidation states, precipitates, colours, identify 3d ions, 3d metals
Many d block compounds give colours in aqueous state. Also d block metal ions forms precipitates with anions such as CO32- , OH-. We can identify these metals or compounds using these precipitates and colours. Some d block metal ions give precipitates with NaOH, ammonia and more solutions. These d block metal hydroxides, sulfates, chlorides and other precipitates and solutions take different colours. d metals have different oxidation numbers such as +2 and +3 for ions. Therefore, Fe(OH)2(s) and Fe(OH)3(s) are green and brown precipitates.
In this tutorial,
we discuss about transition metal compounds and its colours, oxidation states, stable compounds, what are the
precipitates and solutions. Then we list all the transition metal compounds according to the colours and solubility. Finally
we discuss some questions about transition metal compounds.
Colours of d block compounds
Transition metal ions are often coloured. When a single transition metal atom is considered, it's five d orbitals are in same energy level(degenerate).In a complex ion, energy of d orbitals are changed slightly due to overlapping differently with the ligands they are non-degenerate. When d metal ion absorb energy, it's electrons of d orbitals can jump to another d orbit. The frequency absorbed in this energy transitions is in the visible region of the spectrum and the ion appears coloured. The colour of the ion is complementary to the colours absorbed.
Oxidation states of d block metals
The common electron configuration is [Ar] 4s2 3dn. Once the 4s electrons have been removed, the difference in energy between the 3d and the 4s electrons is much smaller than the difference between the 3s and the 3p electrons.
Variable oxidation states
Transition elements exits in several oxidation states. Also oxidation states change in units of one.
Fe2+ and Fe3+ , Cu+ and Cu2+
Stability of various oxidation states
An oxidization state is stable,
- If they exist in room temperature
- are not oxidized by air
- Are not hydrolyzed by water, vapour
- Do not disproportionate / decompose at normal temperature
Rare oxidation states are given inside paranthesis.
Oxidation state of scandium(Sc)
Electronic structure : d1 s2
Oxidation states : +2 , +3
Stable compounds: Sc2O3 , ScF3 , ScCl3 , ScBr3 , ScI3
Oxidation state of titanium(Ti)
Electronic structure: d2 s2
Oxidation states: +2 , +3 , +4
Stable compounds: TiO , TiCl2 , TiBr2 , TiI2 , Ti2O3 , TiO2
Oxidation state of vanadium(V)
Electronic structure: d3 s2
Oxidation states: +2 , +3 , +4, +5
Stable compounds: VO , VF2 , VCl2 , VBr2 , VI2 , V2O3 , VO2 , V2O5
Oxidation state of chromium(Cr)
Electronic structure: d5 s1
Oxidation states: (+1) , +2 , +3 , +4 , +5 , +6
Stable compounds: CrO , CrF2 , CrCl2 , CrBr2 , CrI2 , Cr2O3 , Cr3 , CrO2 , CrF5 , CrO3
Oxidation state of manganese(Mn)
Electronic structure: d5 s2
Oxidation states: +2 , +3 , +4 , +5 , +6 , +7
Stable compounds: MnO , MnF2 , MnCl2 , MnBr2 , MnI2 , Mn2O3 , MnO2 , Mn2O7 , KMnO4
Oxidation state of iron(Fe)
Electronic structure: d6 s2
Oxidation states: +2 , +3
Stable compounds: FeO , FeF2 , FeCl2 , FeBr2 , FeI2 , Fe2O3 , FeF3
Oxidation state of cobalt(Co)
Electronic structure: d7 s2
Oxidation states: +2 , +3 , (+4) , (+5)
Stable compounds: CoO , CoF2 , CoCl2 , CoBr2 , CoI2 , Co2O3
Oxidation state of nickel(Ni)
Electronic structure: d8 s2
Oxidation states: +2 , (+3) , +4
Stable compounds: NiO , NiF2 , NiCl2 , NiBr2 , NiI2 , Ni2O3 , NiO2
Oxidation state of copper(Cu)
Electronic structure: d10 s1
Oxidation states: +1 , +2
Stable compounds: Cu2O , CuCl , CuBr , CuI , CuO , CuF2 , CuCl2 , CuBr2
Oxidation state of zinc(Zn)
Electronic structure: d10 s2
Oxidation states: +2
Stable compounds: ZnO , ZnF2 , ZnCl2 , ZnBr2 , ZnI2
colours of d block metal ions in aqueous state
d block elements can show different oxidation states. As an example Mn has major oxidation numbers +2 , +4 , +6 , +7. When oxidation number changes, the colour of d metal ion in aqueous state changes.
[Sc(H2O)6]3+ - Colourless
[Ti(H2O)6]3+ - Purple
[V(H2O)6]3+ - Green
[Cr(H2O)6]3+ - Violet
[Mn(H2O)6]2+ - Pink
[Mn(H2O)6]3+ - purple
[Fe(H2O)6]2+ - Liht green
[Fe(H2O)6]3+ - Yellow - Brown
[Co(H2O)6]2+ - Pink
[Ni(H2O)6]3+ - Green
[Cu(H2O)6]2+ - Blue
[Cu(H2O)6]+ - Colourless
[Zn(H2O)6]2+ - Colourless
Why scandium(Sc3+) , copper(Cu+) and zinc(Zn2+) ions are colourlesss in aqueous state?
Sc3+ ions don't have d electrones. Therefore it is colourless. Also Cu+ and Zn2+ are with 3d10 configuration. Therefore there is no d to d electrons transition in Cu+ and Zn2+. So Cu+ and Zn2+ ions are colourless.
d block metal hydroxides colours
All of d block metal hydroxides are precipitates and they have different colours. All the d block metals are gelatinous owing to hydration and all are basic. When we add dilute NaOH to d metal ion, a precipitate forms. Some of these hydroxides are amphoteric and some of hydroxides forms soluble complexions with ammonia. Following compounds are some of d metal hydroxides which exist as precipitates and colours of each precipitates are noted.
- Cr(OH)3 - green
- Mn(OH)2 - yellow white
- Fe(OH)2 - green
- Fe(OH)3 - brown
- Co(OH)2 - pink
- Ni(OH)2 - green
- Cu(OH)2 - blue
- Zn(OH)2 - white
d block|transition metal ions with concentrated HCl
When concentrated hydrochloric acid is added to some d block(transition) metal ions(existing as complex compounds in water), they give coloured solutions. Fe2+, Co2+, Ni2+, Cu2+ ions give colours with concentrated HCl.
- [FeCl42-](aq) - green
- [CoCl42-](aq) - blue
- [NiCl22-](aq) - blue
- [CuCl32-](aq) - yellow
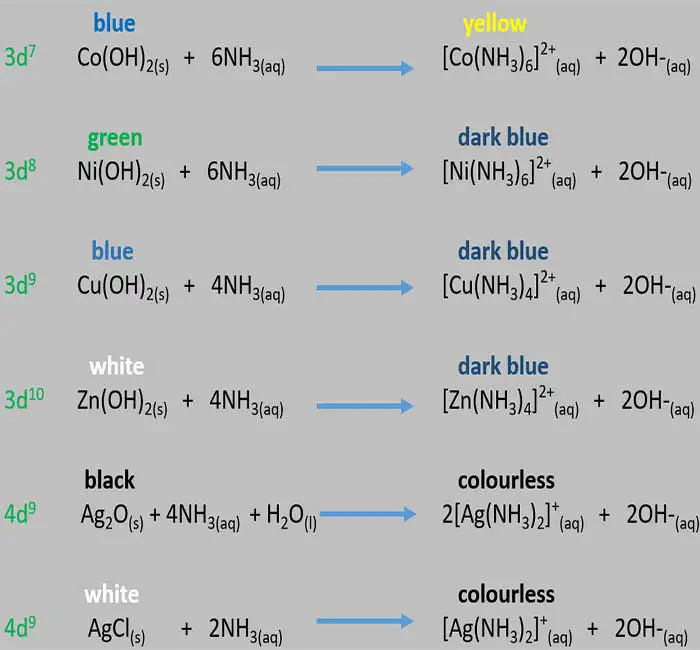
Identify d block cations by ammonia solution
In this lesson, we are going to identify some cations in d block. We can identify d7, d8,d9 and d10 configurations' cations by solving their precipitates in excess ammonia solutions.
When we add excess ammonia solution to precipitates of hydroxides of d block cations,
precipitates dissolve and give different colours related to
different cations.
We can identify Co2+, Ni2+, Cu2+, Zn2+ and Ag+ from this
method.
Sulfides of 3d metal ions
All of d block metal sulfides are precipitates. Some of those sulfides are formed in acidic medium and some are in acidic medium. Hydrogen sulfide (H2S) gas is sent to the 3d block metal ion solutions to form their respective sulfides. Basic medium is made by adding aqueous ammonia solution.
Sulfides which forms in acidic medium and colours
Hydrogen sulfide (H2S) gas is sent to the below acidified d block metal ion solutions to form their respective sulfides
- HgS(s) - black
- Sb2S3(s) - orange
- CdS(s) - yellow
- FeS(s) - black
- SnS(s) - yellow brown
- SnS2(s) - yellow
- CuS(s) - black
Sulfides which forms in basic medium and colours
Hydrogen sulfide (H2S) gas is sent to the below basic d block metal ion solutions to form their respective sulfides
- ZnS(s) - white
- MnS(s) - pink
- NiS(s) - black
- CoS(s) - black
3d metal oxides colours
- Sc2O3: white gelatinous precipitate
- TiO2: white powder
- VO: black
- V2O3: black
- VO2: dark blue
- V2O5: brick red
- Cr2O3: green
- CrO3: dark-purple
- MnO2: brown
- FeO: green
- Fe2O3: brown
- Cu2O: brick red
- CuO: black
Cyanide ions and 3d metal ions complexes
Add potassium cyanide (KCN) solution to d metal ions and check their colours.
- K4[Fe(CN)6](aq) - yellow green
- KFe[Fe(CN)6](s) - prussian blue
- K2Fe[Fe(CN)6](s) - white
- [Ni(CN)2](s) - light green
- K2[Ni(CN)4](aq) - yellow
With thiocyanate ion ( SCN-
- [Fe(SCN)]2+ : blood red
- [Fe(SCN)2]2+ : blood red
- [Fe(SCN)3]2+ : blood red
- [Fe(SCN)4]2+ : blood red
Now, we list all precipitates and solutions according to the colours. It's very useful to remember compounds with colours. This can be used as a short note / summary.
Precipitates of d block metals
Now we summarize all of 3d metal precipitates according to their colours. Exact colour of compound is noted with every precipitate.
Blue colour precipitates
VO2 : dark blue
Cu(OH)2 : light blue
KFe[Fe(CN)6] : prussian blue
KFe[Fe(CN6)] : turnabull blue
[Co(OH)(H2O)5]+ : blue
Green colour precipitates
Cr(OH)3 : dark green
Fe(OH)2 : pale green
Ni(OH)2 : light green
Ni(CN)2 : light green
Yellow colour precipitates
Mn(OH)2 : yellow white
SnS : yellow brown
SnS2 : yellow
CdS : yellow
Black colour precipitates
HgS : black
CuS2 : black
FeS : black
NiS : black
CoS : black
MnO2 : black brown
Aqueous solutions of d block metals
Blue colour solutions
[Cr(H2O)6]+ : blue
[NiCl4]4-(ethanol) : blue
Green colour solutions
K2MnO4 : green
[Fe(H2O)6] : pale green
[Fe(CN)6]4- : yellow green
[Ni(H2O)6]2+ : green
Yellow colour solutions of d metal ions
K2CrO4 : yellow
dilute [Fe(H2O)6]3+ : yellow
[FeCl4]2- : yellow
[Fe(CN)6]4- : yellow green
[Co(NH3)6]2+ : yellow brown
[NiCl4]2- : yellow brown
[Ni(CN)4]2- : yellow
What are the amphoteric compounds of transition metals?
Cr2O3 : green colour precipitate
Cr(OH)3 : a green precipitate
ZnO : white precipitate
Fe2O3 : brown colour precipitate
What are the colours of precipitates of copper(I) ion?
Cu2O : brick red precipitate
CuCl : a white precipitate
CuBr : a white precipitate
CuI : a white precipitate