Acidic and Acidity of Organic Compounds and Organic Chemistry
In this lesson, we learn about acidity of different organic compounds and which organic compounds show acidic characteristics. Acidity of organic compounds depends on many different factors. Some organic compounds are very acidic compared to some organic compounds. Also there are basic organic compounds such as amine compounds.
At the end of this tutorial, you should have the ability to decide which organic compound is more acidic from given compounds and what are the reasons for that. We study all acidic organic compounds in this lesson.
written by: Heshan Nipuna, last update: 29/05/2020
Examples for acidic organic compounds and comparison of acidity
- Alkyne compounds which have acidic hydrogen atom are more acidic than alkynes which do not have acidic hydrogen atoms. As an example, 1-butyne is more acidic than 2-butyne.
- Carboxylic acid compounds are more acidic than alcohol compounds. Example: Ethanol (alcohol) is less acidic than ethanoic acid (carboxylic acid)
Most of the acidic organic compounds are weak acids. Dissociation constant of acids (Ka) value tells us about the acidity or strength of the acid. When Ka value is high, acidic strength is high.
When we study about acidity of compounds, we have to look their reactions with following compounds and products and then observe reaction rates.
Acidic organic compounds react with one or more following metals or bases to show acidic characteristics
- With sodium or potassium - We check hydrogen gas is eliminated or not.
- With aqueous NaOH solution or aqueous KOH solution - Give a salt and water as products.
- Aqueous sodium bicarbonate (Na2CO3) solution or aqueous sodium bicarbonate (NaHCO3) solution - They are weak bases. To react with these weak bases, acidity strength of organic compound should be high.
Acidic strength of organic compounds
Following table illustrates acidity of various kinds of organc compounds. Later on this tutorial, you will learn why these compounds show acidic properties and why acidic strength is different.
| Organic compound type | Acidity |
|---|---|
| Alkanes | No acidic characteristics |
| Alkenes | No acidic characteristics |
| Alkynes with acidic hydrogen | has weak acidic characteristics, reacts with sodium |
| Alkynes (no acidic hydrogen) | No acidic characteristics |
| Benzene | No acidic characteristics |
| Alkyl halides | No clear acidic characteristics |
| Alcohols | has weak acidic characteristics, acidic than alkynes |
| Phenol | has weak acidic characteristics, acidic than alcohols |
| Aldehyde | Acidic, reacts with strong alkali such as NaOH |
| Ketone | Aldehydes with less molecular mass are soluble |
| Carboxylic acid |
|
| Carboxylic acid chlorides | Reacts with water and form soluble strong acid (HCl) and a weak acid (carboxylic acid). |
| Amides | Basic. |
| Amines | Less basic than amines. |
Acidity of alkane, alkene and alkyne - Hydrocarbons
Alkane, alkene and alkyne are hydrocarbons and there are different characteristics of acidity of these compounds.
Alkyne
Alkyne compounds which contain acidic hydrogen react with reactive metals such as sodium or potassium and H2 gas is emitted as a product. But those alkyne do not react with sodium hydroxide.
Acidity of propyne and 2-butyne
When we look their structure, it is clear that 2-butyne does not have acidic hydrogen. But propyne has a acidic hydrogen atom. Therefore propyne is more acidic than 2-butyne.
Alkane and Alkene
Alkane and alkene do not react with sodium or sodium hydroxide to show acidic properties. However alkyne with acidic hydrogen has some acidic properties than alkane and alkene because alkyne with acidic hydrogen react with sodium.
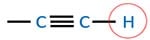
Benzene
Benzene does not react with sodium. That means acidity of benzene is below than alkyne.
Also benzene does not react with aqueous sodium hydroxide, sodium carbonate and sodium bicarbonate.
Alkyl halide compounds
Alkyl haide compounds react with aqueous NaOH or KOH to give alcohols. But this reactions does not belong to acid base reaction type. In this reaction, halogen atom of alkyl halide is replaced by hydroxyl group of NaOH or KOH.
Alcohols
Alcohols react with sodium and emit hydrogen gas. It says alcohols are acidic organic commpounds.
But alcohols do not react with aqueous sodium hydroxide to give the salt and water. Also alcohols do not react with aqueous sodium carbonate and sodium bicarbonate whcig are weak bases..
Ethanol and methanol acidity
pKa of ethanol = 16
pKa of methanol = 15.5
Lower the pKa value, acidic strength increases. So methanol is more acidic than ethanol.
Phenol
- Phenol reacts with sodium and emit hydrogen gas.
- Also phenol reacts with aqueous NaOH. From this reaction, we can say, phenol is more acidic than alcohol.
- But phenol does not react with aqueous Na2CO3 or NaHCO3.
Phenol is more acidic than alcohols.
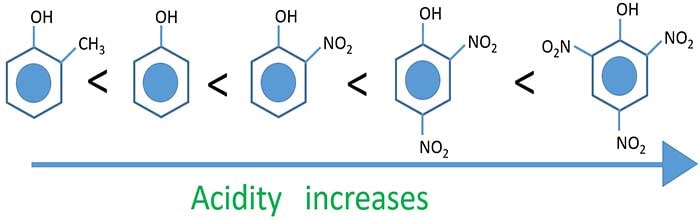
Acidity of phenol derivatives
Acidity depends on polarity of O-H bond. When polarity of O-H bond increases, acidity also
increases.
Electron density of -O-CH3 is high. Therefore it gives electrons to the benzene ring which decreases
polarity of O-H bond.
Electron density of -NO2 is less. Therefore it attracts electrons from benzene ring. Then O-H bond gives
electrons to the ring which increases polarity of O-H bond. When more NO2 groups connect to the ring,
polarity of O-H bond more increases.
Carboxylic Acids
Aqueous carboxylic acids are much acidic than alcohols and phenol.
- Carboxylic acids react with sodium and emit hydrogen gas.
- Also carboxylic acids react with aqueous NaOH and produce sodium carboxylate and water.
- Carboxylic acids react with aqueous Na2CO3 or NaHCO3 and emit CO2 gas.
Due to reaction with weak bases such as Na2CO3 or NaHCO3, we can say carboxylic acids are acidic than alcohols nd phenol.
Carbonic acid | H2CO3
Carbonic acid is a weak organic acid and a dibasic acid. Carbonic acid can release two hydrogen ions in aqueous state. It's pK value is 6.4 .
Methanoic acid and carbonic acid
pKa of methanoic acid (formic acid) is 3.75. Therefore methanoic acid is much acidic than carbonic acid.
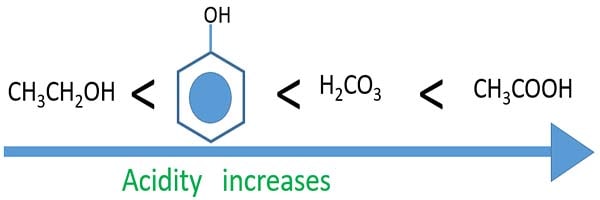
Acidity of alcohols, phenol, carbonic acid and carboxylic acid
- All of those acids react with Na and H2 gas is emitted.
- Phenol, carbonic acid and carboxylic acids react with NaOH. Only alcohol compounds do not react with NaOH. Therefore alcohols are less acidic than other compound types.
- Solid Na2CO3 and NaHCO3 react with carboxylic acids and emit CO2.
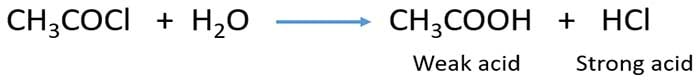
Acidity of acid chlorides
When acid chloride is added into the water, acid chloride (ex: CH3COCl )
hydrolyses into strong acid ( HCl ) and carboxylic acid ( CH3COOH ).
Due to exist of strong acid in the aqueous solution it is more acidic than aqueous carboxylic acid solution.
Questions
does alkene have a strong acid compared to alkanes?
No, Both alkanes and alkenes do not show acidic properties. Therefore, No strong acids of alkanes and alkenes.
which compound is most acidic benzene,alkene
Both solutions are do not show specific acidic characteristics. Benzene and alkene do not react with sodium, aqueous sodium hydroxide, aqueous sodium carbonate, aqueous sodium bicarbonate. Usually acidic organic compounds (alcohols, phenol, carboxylic acids) react with one or more of sodium, aqueous sodium hydroxide, aqueous sodium carbonate, aqueous sodium bicarbonate.
From following organic compounds, which is most acidic? List them according to the acidic strength.
- propanol
- Propanoic acid
- Propyne
All are organic compounds with three carbon atoms. But each compound belongs to different types of organic compounds.
- propanol: alcohol
- Propanoic acid: carboxylic acid
- Propyne: alkyne which contains acidic hydrogen
Acidic strength increases as like this, propyne < propanol < propanoic acid
Acidity of 4-nitrophenol than phenol
When a nitro group is attached to the benzene ring, it attracks the electrons of benzene ring ( act as an deactivator). Also this attraction effects to the -OH group and increases the polarity of hydrogen atom. Therefore acidity of 4-nitrophenol is higher than phenol.
Which of the following organic compounds is most acidic?
- C2H4
- CH3CCH
- CH3OH
First, identify the type of organic compounds. C2H4 is an alkene and CH3CCH is an alkyne with an acidic hydrogen. So CH3CCH is more acidic than C2H4.
CH3OH is an alcohol and it is more acidic than CH3CCH.
Are there strong acidic organic compound such as HCl or H2SO4
Most of the acidic organic compounds are weak acids. But acidic strength depends on the types of organic compound. Carboxylic acids are much acidic than other acidic organic compounds. But carboxylic acids are weak acids too.
References
pKa Values of Organic and Inorganic Acids
Writer: Heshan Nipuna, Department of Chemical and Process Engineering, University of Peradeniya
Published: 14 September 2017
Last edit: 04 January 2020