Preparation of Benzene (C6H6) in Laboratory and Industrial Scale
Benzene (C6H6) is produced as a by-product of petroleum industry. As well benzene can be produced from some other chemicals in a chemical laboratory and methods of benzene preparation are explained in detail in this tutorial.
Content
- Preparation of Benzene in laboratory
- Preparation of benzene from acetylene
- Preparation of benzene from benzoic acid
- Preparation of benzene from Phenol
- Preparation of benzene from diazonium salts
- Industrial preparation of benzene
- Catalytic reforming
- Hydrodealkylation of Toluene
Preparation of Benzene in laboratory
Benzene can be prepared by several chemicals in the laboratory. But, we have to be careful when benzene is being produced due to it's toxicity. Therefore, follow safety protocols thoroughly to avoid short-time and longtime effects to the health.
- Preparation of benzene from acetylene
- Preparation of benzene from benzoic acid
- Preparation of benzene from Phenol
- Preparation of benzene from diazonium salts
- Prepare benzene from Grignard reagent hydrolysis
- Preparation of benzene from aryl halides with Ni-Al alloy in the presence of NaOH
- Preparation of benzene from sulphonic acid
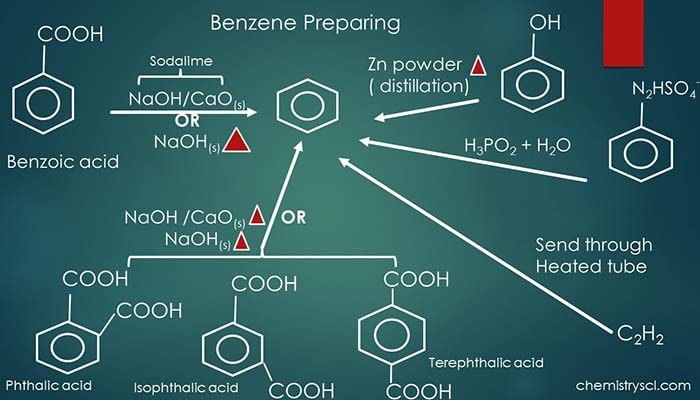
Prepare benzene from acetylene | acetylene condensation
Alkyne compounds such as acetylene (ethylene) polymerizes at high temperature to give arene compounds such as benzene.
Send Acetylene gas through a hot iron pipe. Here, benzene is prepared as a non aromatic compound. Three acetylene moles joins together in the hot iron tube with catalyst condensation. This reaction was first invented by Berthelot.

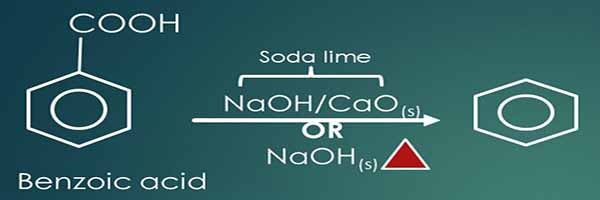
Preparation of benzene from benzoic acid
Benzoic acid is an aromatic carboxylic acid compound. When benzoic acid is mixed with soda lime or solid Sodium hydroxide and heated, Benzene is given as a carboxylic acid group reduction. A mixture of Calcium oxide and Sodium hydroxide is used as soda lime mixture.
Prepare benzene from Phenol
Phenol is an aromatic alcohol compound. When Phenol is heated with Zinc dust, -OH group is eliminated and benzene is given as the product. Zinc is oxidized to Zinc oxide (ZnO) during this reaction.

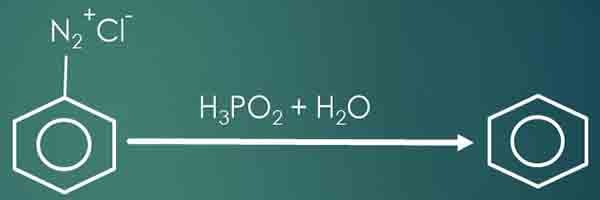
Prepare benzene from Benzene diazonium chloride
Benzene diazonium chloride is an unstable compound at room temperature. However, Benzene diazonium chloride can be used to prepare benzene in two ways.
- Benzene diazonium chloride and hypophosphorus acid (H3PO2) in the presence of Cu(I) ions. In this reaction, Benzene, Nitrogen gas, Hydrochloric (HCl) acid and Phosphorous acid (H3PO3) are given as products.
- Benzene diazonium chloride and ethanol (CH3CH2OH) reaction.
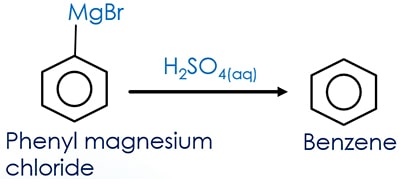
Prepare benzene from Grignard reagent hydrolysis
When Grignard reagent is exposed to water or dilute acid solution, Grignard reagent readily hydrolysis to respective alkane compound. In this reaction too, we use the same concept.
Hydrolysis of any phenyl magnesium halide in acidic medium will give benzene as the product. If dilute sulfuric acid and phenyl magnesium chloride are mixed together, benzene is given as the product.
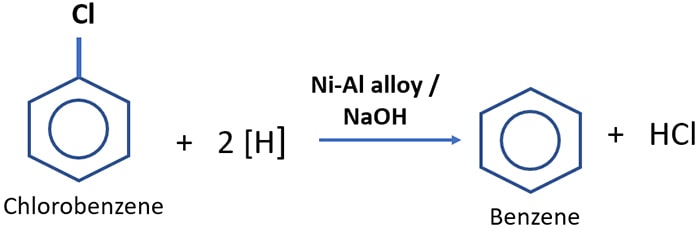
Preparation of benzene from aryl halides with Ni-Al alloy in the presence of NaOH
Aryl halide compounds are defined as, when a halogen atom is directly joint to the benzene ring. Benzene is prepared by the reaction of chlorobenzene and Ni-Al alloy in the presence of Sodium hydroxide.
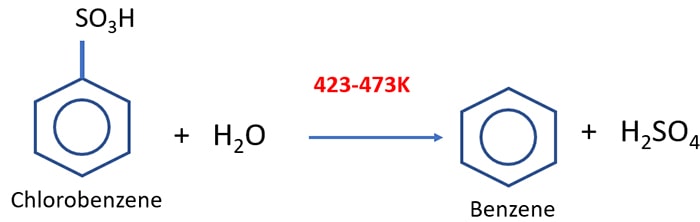
Preparation of benzene from sulphonic acid
When Benzene sulphonic acid is heated with superheated steam (423K - 473K), benzene is given as a product. As a by-product, Sulfuric acid is formed.
Summary of Benzene preparing in the laboratory
Industrial preparation of benzene
Industrial preparation of benzene is based on the crude oil refining process.
- Catalytic reforming
- Toluene hydrodealkylation
- Steam cracking
Catalytic reforming
Preparation of benzene by catalytic reforming is explained step by step.
- First, a mixture of hydrocarbons are blended with hydrogen gas between 60-2000C temperature.
- Then, above gas mixture is exposed to catalyst (Platinum(II) chloride (PtCl2) or Rhenium(III0 chloride (RhCl3) ) at 8-50 atm.
- Now, aliphatic hydrocarbons are converted to aromatic hydrocarbons including benzene. So, there are several aromatic compounds in the product range.
- As the next step, aromatic hydrocarbons are separated from the product mixture by an extraction process using solvents (diethlene glycol or sulfolane).
- Finally, as the purification and separation of benzene, aromatic product range is undergone a distillation process.
Hydrodealkylation of Toluene
In this process too, a catalytic conversion reaction is applied at medium temperature and medium pressure conditions.
- As the raw material, Toluene and hydrogen gas are used.
- As catalyst, Chromium or Molybdenum or Platinum oxide are used and Tolene and hydrogen gas mixture is passed over the catalyst layer at 500-6000C at 40-60 atm pressure conditions.
- Then, -CH3 group of Toluene is eliminated from the benzene ring and Benzene and methane are given as products.
Have Questions?
Is benzene a toxic compound?
Yes. Benzene is toxic compounds. It has benn found that benzene causes to cancers. Due to this toxicity of benzene, use of it is limited. Toluene is used instead of benzene.
What is the shape, bond length, angle of benzene
According to the X-rays diffraction, it is found that shape of benzene is analogous hexagon. Bond angle of C-C-H is 1200C. All C-C bond lengths are same(0.139mm).
how to synthesis salt using different benzene numbers
Question is not clear. As salts which contains a benzene group we can list sodium benzoate, sodium phenate.