Ethanol to Butyl ethanoate (butyl acetate) - Ester preparing
Ethanol is an alcohol compound and butyl ethanoate is an ester compound. Esters can be prepared by following reactions.
- Reaction of alcohol compound and carboxylic acid in the presence of concentrated sulfuric acid
- Reaction of carboxylic acid chloride and alcohol compound
More to read: ester preparing reactions
Convert ethanol to butyl ethanoate
Synthesis butyl ethanoate by using chemical reagents. But you have to start the conversion from ethanol.
Butyl ethanoate molecule
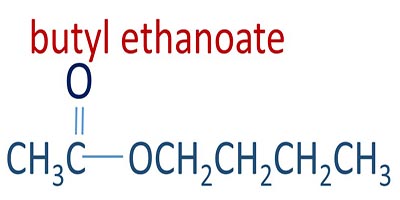
Preparation of butyl ethanoate
Ethanol has only two carbon atoms. But butyl ethanoate has six carbon atoms in its molecule. So, we can understand we have to do a reaction which will extend the carbon chain. In this problem, we use aldol condensation to extend carbon chain. For aldol condensation, we have to prepare an aldehyde or ketone.
Steps of this conversion are explained below.
How an ester is prepared?
So we know there should be alcohol and carboxylic acid chloride to give the ester. Our alcohol compound should have four carbon atoms and carboxylic acid chloride should have two carbon atoms. Therefore we have to go in two ways to prepare corresponding alcohol and carboxylic acid.
Oxidizing ethanol to ethanoic acid
Alcohols are oxidized by strong oxidizing agents such as H+ / KMnO4, H+ / K2CrO4, H+ / K2Cr2O7 to give carboxylic acids. So, ethanol is oxidized to ethanoic acid.
Ethanoic acid to ethanoyl chloride
Then ethanoic acid reacts with PCl3 / PCl5 to give carboxylic acid chloride, ethanoyl chloride.
Aldol condensation to extend carbon chain
Ethanol to ethanal
Ethanol reacts with PCC to give ethanal. Ethanal is an aldehyde compound.
Aldol condensation of ethanal
Next, ethanal reacts with dilute NaOH or KOH. This reaction is the aldol condensation. Then given mixture is heated to prepare 2-butenal which has an alkene group and carbonyl group. Next, 2-butenal reacts with H2 in the presence of catalyst. It hydrogenize alkene group to alkane and convert aldehyde group to alcohol group. It gives butanol as the product.
Finally ethanoyl chloride reacts with butanol to give our product, butyl ethanoate. In this reaction, HCl is produced as a by product.
Conversion of ethanol to butyl ethanoate
Following figure shows, how butyl ethanoate is prepared by ethanol step by step.
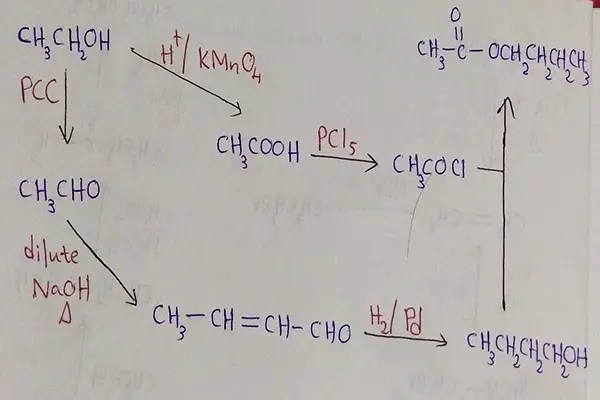
Butyl ethanoate reaction
Butyl ethanoate shows reactions of ester compounds. Reducing esters, hydrolysis of esters are some example reactions.
Butayl ethanoate hydrolysis reaction
Butayl ethanoate can be hydrolyzed by concentrated sulfuric acid or aqueous strong bases such as NaOH, KOH.