Ethanol to Ethane, Bromoethane, Ethene
Both ethanol and ethane has two carbon atoms and ethnol can be converted to ethane through preparing ethene or grignard reagent. Ethanol is a liquid compound in room temperature and ethane is a gas in room temperature.
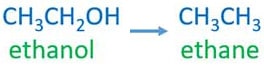
Ethanol and ethane
Ethanol - CH3CH2OH - an alcohol compound
Ethane - CH3CH3 - an alkane compound
Ethanol to ethane
Ethanol to ethane is done by two ways in this tutorial.
- through grignard reagent
- through ethene (alkene)
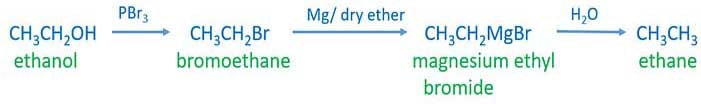
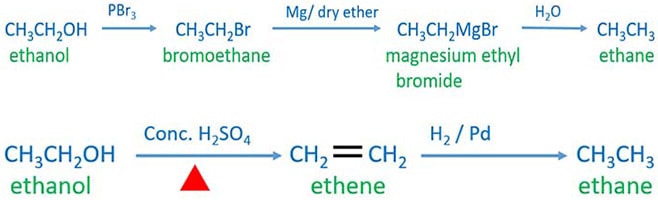
First we discuss ethanol to ethane by preparing bromoethane, and grignardd reagent.
Ethanol to ethane through grignard reagent
Preparing bromoethane from ethanol
Add PBr3 to ethanol. That will give bromoethane. Also HBr can be used to prepare bromoethane. HBr reacts with ethanol to give bromoethane.
Preparing grignard reagent
Add Mg and dry ether to bromoethane. ethyl magnesium bromide(CH3CH2MgBr) is formed.
Grignard reagent and water reaction
Grignard reagent and water reaction will give ethane.
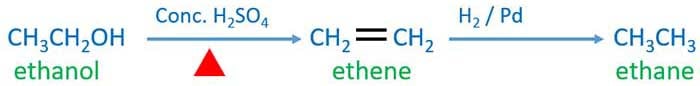
Ethanol to ethane through ethene
In this method, we prepare ethene from ethanol. Then ethane is prepared by ethene.
Ethene preparation from ethanol
Ethene is prepared by adding concentrated sulfuric acid to ethanol and heating the mixture. Other dehydrators such as Al2O3, P2O5, H3PO4 are also can be used insted of concentrated sulfuric acid to prepare ethene from ethanol. Ethene (CH2-CH2) is an alkene compound which has two carbon atoms.
Ethene to ethane
Then prepared ethene and H2 with in the presence of Pt catalytic are reacted. That will give ethane as the product.
Ethanol to ethane by preparing ethanal
We can convert alcohol to aldehyde. Then aldehyde is converted to alkane.
Ethanol to ethanal
Ethanol reacts with PCC to give ethanl. Ethanal is an aldehyde compound. ( ethanal - CH3CHO ). PCC is pyridinium chlorochromate which is used to oxidize alcohols to aldehydes.
Ethanal to ethane
Ethanal is reduced to ethane by clemmensen reduction. Zn(Hg) and concentrated HCl is used as clemmensen reagent. Aldehydes and ketones are reduced to hydrocarbons by clemmensen reduction reaction.
Clemmensen reduction reactionIdentify ethanol and ethane and separating
Ethanol is a liquid in room temperature. But ethane( an alkane compound) is a gas at room temperature. Ethanol reacts with Na and emits H2 gas and ethane does not.
ethanol to ethane summary
Questions asked on ethane preparation
Is there a phase change in ethanol to ethane
Ethanol is a liquid in room temperture. But ethane is a gas. So there is a phase change, liquid to gas in ethanol to ethane.
How is ethene prepared from ethanol and give the reaction involved in it
Ethene is prepared from ethanol from one step. Ethanol is heated with concentrated sulfuric acid to prepare ethene. As sulfuric acid, another dehydrators can be used.
Dehydrators used in ethanol to ethene
- concentrated H2SO4 + heating
- Al2O3 + heating
- heated P2O5
- concentrated hot H3PO
What is more reactive, ethanol or ethane?
Alcohols are more reactive than alkanes. Therefore ethanol is more reactive than ethane.
How will you convert ethane into ethanol give the chemical reaction involved
Ethane is an alkane compound which are very inactive. If we can convert ethane to bromoethane by brominization under sun light, we can prepare ethanol. Bromethane reacts with dilute NaOH to give ethanol.
How is ethene prepared from bromoethane?
Bromethane is heated with alcoholic KOH to prepare ethene.
How is ethene prepared from ethanal?
Ethanal is reduced to ethanol by LiAlH4. Then ethanol is dehydrated from acidic concentrated sulfuric acid to prpare ethene.
Is it possible to convert ethane to ethanol?
We know alkanes are not reactive like alcohols. So it is not easy to convert ethane to ethanol like ethanol to ethane. If we make chlorooethane from ethane we can make ethanol. We can prepare chloroethane as one product from alkane chlorination reaction. But this is not a good way because it is a mixture of many compounds and we have to separate chloroethane from that mixture.