>Ethanol to butane, Preparation of butane
Butane is an alkane compound and ethanol is an alcohol. Butane includes four carbon atoms and ethnol has two. Butane can be prepared by ethanol several methods. Finally, we discuss how butane and ethanol compounds are separated.
Chemical Formulas of ethanol and butane
- Ethanol - CH3CH2OH
- Butane - CH3CH2 CH2CH2
Question
Synthesis butane by starting from ethanol. You can only use ethanol as an organic compound. You can choose any other reactant for this conversion.
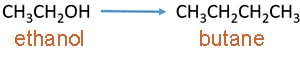
Preparation of butane
- prepare butane from ethanol through acetylene
- prepare butane from ethanol using aldol condensation
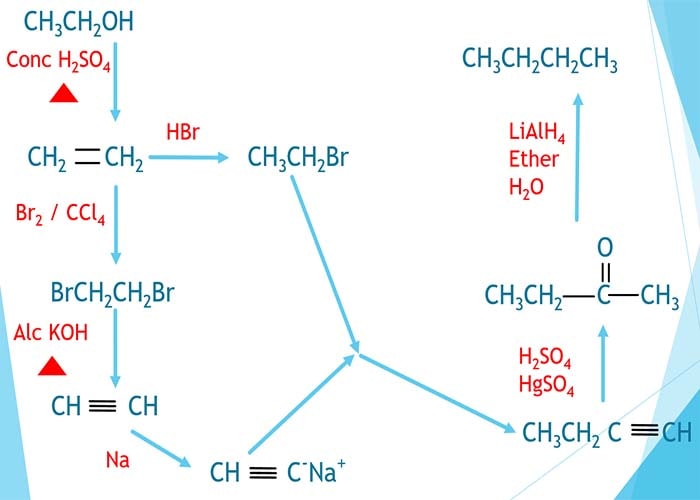
Prepare butane by ethanol through acetylene
Ethanol and butane contain two and four carbon atoms respectively. We can assume two carbon chains are aggregated to form butane. Here, those two carbon chanis are prepared by ethanol here.
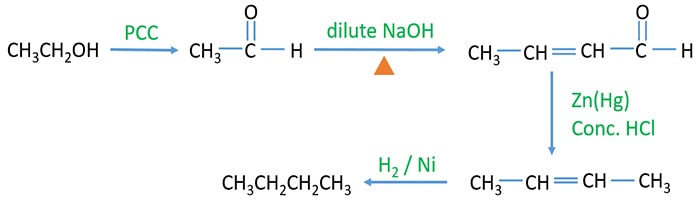
Prepare butane by ethanol through aldol condensation
- First, ethanol react with PCC (Pyridinium chlorochromate) and give CH3CHO (aldehyde).
- Then, CH3CHO reacts with dilute NaOH and heat the mixture, and it gives CH3CHCHCHO. This is aldol condensation.
- Next, Given product is reduced by clemmensen reduction. For clemmensen reduction, Zn(Hg) and concentrated HCl is used as the reagent. This will reduce aldehyde group to hydrocarbon group and new product is an alkene now (CH3CH=CHCH3).
- Now, Send H2 to the CH3CHCHCH3 in the presence of Ni catalyst and it gives the required butane.
Identify ethanol and butane
Ethanol is a liquid at room temperature ( 250C ). But butane is a gas at room temperature Boiling point of ethanol is higher than butane. Ethanol react with Na and emits H2(g). But butane does not react with Na.
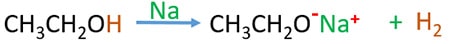
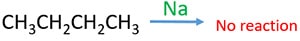
Questions asked on ethanol and butane
Still have a Question? Ask your question now. We will help you.How is ethanol obtained from 2-butene
2-butene contains four carbon atoms and double bond is located bwtween second and third carbon atoms. Therefore it is symmetric around double bond. We can brake 2-butene molecule through double bond using acidic KMnO4. There we get two ethanoic acid molecules from one 2-butene molecue. Then ethanoic acid is reduced to ethanol by LiAlH4.
Preparation of butane
The alkenes, 1-butene, 2-butene can be used to prepare butane. These alkenes react with Pt / H2 to prepare butane.
Butanal ( the aldehyde ) reacts with Zn (Hg) and concentrated HCl to give butane. This is clemmensen reduction of butanal
Is butane an alcohol compound?
Butane is an alkane compound. Therefore there are only single bonds between carbon atoms.
Is ethanol a compound?
Ethanol is a covalent compound. It contains carbon, hydrogen and oxygen atoms.
How is ethanol obtained from 2-butene?
2-butene is an alkene. When alkene reacts with H2 in the presence of Ni or Pt or Pd catalyst, alkanes are given. Therefore 2-butene can be converted into butane by hydrogenation reaction.