Ethanol to Eutanoic acid | Ethanol + Butanoic Acid Reaction | Butanol to Butanoic Acid
Here, we are going to synthesis butanoic acid from ethanol and learn the reaction between ethanol and butanoic acid.
Question
Preparing butanoic acid from ethanol
Derive butanoic acid by starting from Ethanol. You can only use ethanol as an organic compound and following reagents during the conversion.
Other reactants : Na , Concentrated H2SO4 , H+/KMnO4 , Br2 , Pd/BaSO4/Quinolene , dilute NaOH , organic peroxides , HBr , Alcoholic KOH

In this tutorial, we study about
- Preparation of butanoic acid from ethanol
- Butanoic acid + ethanol reaction
- Questions of ethanol and butanoic acid
Ethanol to butanoic acid
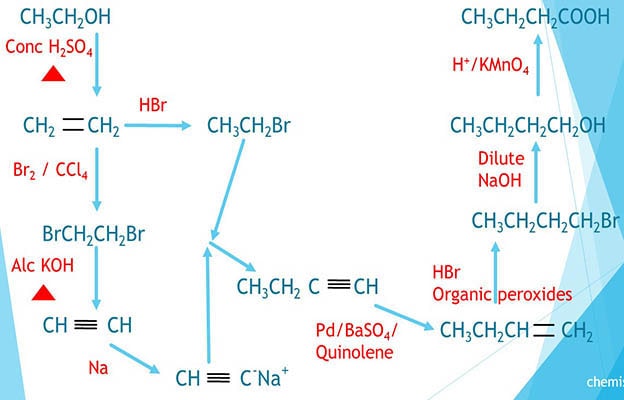
Steps of preparation of butanoic acid from ethanol is explained below.
- Butanoic acid is a carboxylic acid compound including four carbon atoms.
- But ethanol, an alcohol compound which only contains two carbon atoms in its structure.
- Therefore we have to extend the carbon chain when preparing butanoic acid from ethanol.
Chemical Formula
- Ethanol - CH3CH2OH
- Butanoic acid - CH3CH2CH2COOH
Ethanol to ethene
Ethanol is heated with concentrated sulfuric acid to give ethene. Ethene is an alkene compound.
Ethene to 1-2-dibromoethane
Ethene reacts with liquid bromide ( Br2 / CCl4 ) and give 1-2-dibromoethane compound which is an alkyl halide.
1-2-dibromoethane to ethyne
1-2-dibromoethane is heated with alcoholic KOH to get ethyne.
Ethyne and sodium reaction
Less amount of Na is added to ethyne. Sodium salt of alkyne is formed. This can be used to extend carbon chain.
Ethene to bromoethane
Ethene reacts with HBr and give bromoethane (ethyl bromide), an alkyl halide compound.
Extending carbon chain
Sodium salt of alkyne and bromoethane react to give the compound with extended carbon chain. That is 1-butyne. This is also an alkyne compound with four carbon atoms.
1-butyne to 1-butene
1-butyne reacts with H2 / Lindlar's catalyst to give 1-butene. Lindlar's catalyst is used to stop hydrogenation upto alkanes.
1-butene to 1-bromobutane
1-butene reacts with HBr in the presence of organic peroxides to give 1-bromobutane.
1-bromobutane to 1-butanol
1-butanol is given by 1-bromobutane with reaction of dilute NaOH.
1-butanol to butanoic acid
Butanol is oxidized to butanoic acid by acidic potassium permanganate ( H+ / KMnO4 ).
Ethanol + butanoic acid
Ethanol is an alcohol compound. Butanoic acid is a carboxylic acid. Therefore in the presence of concentrated H2SO4, ester product is given. This reaction is a reversible one. Ester product is ethyl butanoate.
Questions about ethanol and butanoic acid asked by students
What is the reaction of ethanol and butanoic acid
Ethanol is an alcohol and butanoic acid is carboxylic acid. Ethanol, butanoic acid reacts when in the presence of sulfuric acid. It gives the ester, ethyl butanoate as the product.
Ethanol + butanoic acid reaction is possible?
Ethanol + butanoic acid reactions happens in considerable amount if only in the presence of concentrated sulfuric acid. This reaction is a reversible one. Ethanol and butanoic acid reaction form a pleasant smell due to formation of ester.
Butanol to butanoic acid
As primary alcohols are oxidized to carboxylic acids by strong oxidizing agents. So, butanol can be oxidized to a carboxylic acid compound.
Butanol is a primary alcohol and is oxidized to butanoic acid by following reagants. These are very strong oxidizing agents.
- H+/ KMnO4
- H+/ K2CrO4
- H+/ K2Cr2O7
Is ethanol and butanoic acid is an acid - base reaction?
No. Ethanol reacts with butanoic acid in the presence of concentrated H2SO4 acid and give ethyl ethanoate as ester and water as products. If an acid reacts with a base, a salt and water are the products. But ester is not a salt. Therefore ethanol + butanoic acid reaction is not a acid - base reaction.
Butanoic acid formula?
'But' suffix says there are four carbon atoms in the main chain of organic molecule. No substitute groups are in the butanoic acid. So only four crbon atoms in the molecule. So, we can write the molecular formul as, C4H8O2.
Butanoic acid condensed formula?
CH3CH2CH2COOH
How is butanoic acid prepared from an alcohol?
As earlier decribed in this tutorial, other alcohols such as propanol or methanol can be used to prepare butanoic acid.
As butanoic acid from butanol, can I prepare butanol from butanoic acid?
When Butanoic acid is reduced by LiAlH4, butanol is given as the alcohol.
butanoic acid from butene?
If we can prepare butanol from butene, we can prepare butanoic acid. So how to prepare butanol from butene?
Butene to butanol
- Butene reacts with HBr in the presence of organic peroxide. Which will results, 1-bromobutane.
- Then add aqueous NaOH to 1-bromobutane to produce butanol.
Then, butanol is oxidized by a strong oxidizing agent to get butanoic acid as the final product.
How will you prepare butanoic acid from an alcohol?
Butanoic acid is a carboxylic acid. If you want to prepare a carboxylic acid from an alcohol, it should be a primary alcohol. So butanoic acid has four carbon atoms. Then we know we want a primary alcohol which has four carbon atoms. Butanol is the primary alcohol which has four carbon atoms.
By reacting with strong oxidizing agent, butanol is oxidized to butanoic acid.
butanoic acid and methanol reaction?
To butanoic acid react with methanol, we have to add concentrated sulfuric acid and have to supply heat. As the product, methyl butanoate (an ester) is given.
given 8.55g of butanoic acid and excess ethanol
Molecular mass of butanoic acid = 12*4 + 1*8 + 16*2
Molecular mass of butanoic acid = 88 g mol-1
Amount of butanoic acid = 8.55 g / 88 g mol-1
Amount of butanoic acid = 8.55 g / 88 g mol-1
Amount of butanoic acid = 0.1 mol