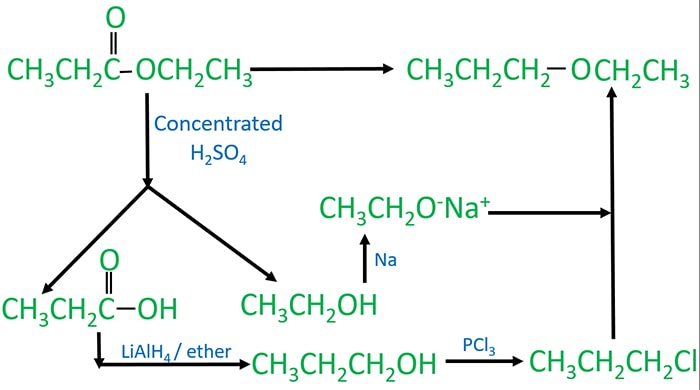
Ethyl propyl ether from ethyl propanoate
Ethyl propanoate is an ester compound and ethyl propyl ether is an ether compound. Therefore we have to brake (hydrolysis of ether) ester to different compounds to prepare ether.
Esters hydrolysis can be done by using an acid or alkali.
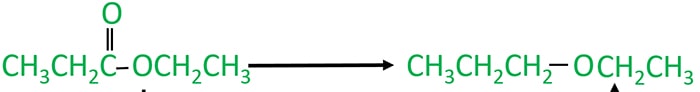
You can see in both molecules are different in their structures. As a organic compound, we can only use ethyl propanoate.
Ethyl propyl ether molecule
There are five carbon atoms in ethyl propyl ether.
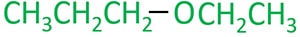
Ethyl propanoate molecule
Ethyl propanoate is an ester compound which can be prepared from reaction of an acyl chloride and an alcohol.
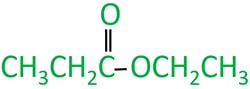
Ethyl propanoate to ethyl propyl ether
Steps of preparing ethyl propyl ether from ethyl propanaote are explained below. n this section you will learn about ester hydrolysis and ether preparing.
How to prepare an ether compound?
Ether is prepared by reaction of sodium salt of alcohols and alkyl halide. Sodium ion and halide ion eliminated to give NaX (X=Cl, Br, I) while ether is produced.
Ester hydrolysis
Ester hydrolysis can be done in two ways, acidic or alkaline.
Ethyl propanoate acidic hydrolysis
Ethyl propanoate hydrolysis is done by concentrated sulfuric acid, which is acidic hydrolysis. It gives ethanol (alcohol) and propanoic acid. But remember that, this reaction is reversible and does not occur 100% hydrolysis.
Ethyl propanoate alkali hydrolysis
With aqueous NaOH, ethyl propanoate hydrolysis will give ethanol and sodium propanaote. To recover propanoic acid from sodium propanaote, add concentrated HCl solution.
Ethanol and propanoic acid separating
Ethanol and propanoic acid can be separated by a physical separation method because their physical properties are different.
Ethanol and sodium reaction
Separated ethanol reacts with sodium metal (Na) to give sodium ethoxide which is a strong base.
We cannot use aqueous NaOH to prepare sodium ethoxide.
Propanoic acid and LiAlH4 reaction
Propanoic acid is reduced to propanol by LiAlH4.
Propanol and PCl3 reaction
Propanol reacts with PCl3 to chloropropane which is an alkyl halide compound.
Also wwe can use PCl5, SOCl2 to prepare chloropropane.
As chloropropane, bromopropane can be used in this conversion to prepare ethyl propyl ether.
Chloropropane and sodium ethoxide reaction
Ethyl propyl ether is given as the product by the reaction of chloropropane and sodium ethoxide. NaCl is eliminated to give the product. No other reagent is required to occur this reaction.