Propanol to Propanone | 2-Propanol Oxidation
Propanone is the simplest ketone compound in organic chemistry. Propanone can be prepared by following ways.
- Oxidation of 2-propanol (secondary alcohol oxidation).
- Hydration of propyne (alkyne)
But, as the organic reagent, propanol is given. Propanol is a primary alcohol and oxidation of propanol will not give propanone as the product after oxidation.
Oxidation of primary alcohol will give aldehyde or carboxylic acids as products. Given product depends on the oxidizing agent is strong or weak.
So we have to convert propanol to 2-propanol or propyne.
Propanol to 2-propanol
Following steps are done to get 2-propanol.
Dehydration of propanol to propene
Propanol can be dehydrated by dehydrators such as concentrated sulfuric acid or alumina or
diphosphorous pentoxide. When propanol is heated with one of these dehydrator, propene is
given as the product.
Hydration of propene to 2-propanol
With dilute sulfuric acid, propene is hydrated. As the product, 2-propanol is given.
Oxidation of 2-propanol
With strong oxidizing agents, propanol is readily oxidized to propanone. For oxidation, strong or mild oxidizing agent is used.

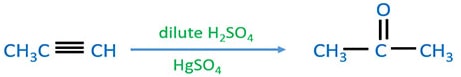
Hydration of propyne
When propyne is hydrated by using dilute H2SO4 and HgSO4, propanone is given as the product.
How propyne is prepared from propanol?
Propanol can be converted to proyne in several steps.
- As learnt before, propene is prepared by propanol.
- Then, propene reacts with liquid bromine to prepare 1,2-dibromopropane, an alkyl halide compound.
- Then, given alkyl halide (1,2-dibromopropaane) is heated with alcoholic KOH to prepare propyne.