Preparation of Phthalic Acids - Phthalic, Isophthalic, Terephthalic Acids Preparing
When two carboxylic groups are substituted to benzene ring, phthalic, isophthalic, terephthalic acids are given. According to the substituted location (ortho, para and meta), those acids are names as phthalic, isophthalic, terephthalic.
Phthalic acid preparing
Alkyl benzene compounds heated with strong oxidizing agents. Then alkyl group is oxidized to carboxylic acid group. When two alkyl groups are oxidized, two carboxylic acid groups are given.
Alkyl benzene are oxidized to phthalic acids by following strong oxidizing agents.
- Acidic potassium permanganate
- Acidic potassium dichromate
- Acidic potassium chromate
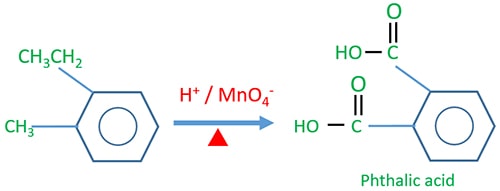
Phthalic acid preparing
In phthalic acid, one -COOH group is connected relatively to the ortho position of other -COOH group.
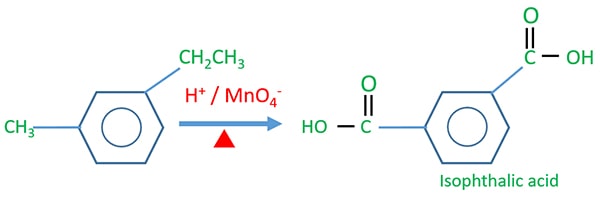
Isophthalic acid Preparing
In isophthalic acid, one -COOH group is connected relatively to the meta position of other -COOH group.
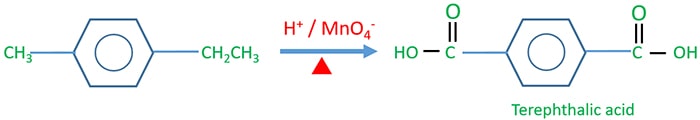
Terephthalic acid Preparing
In terephthalic acid, one -COOH group is connected relatively to the para position of other -COOH group. Terephthalic acid is used to produce teriline, a polymer.
Preparing phthalic acids from benzene
If we need to prepare phthalic acid from benzene, we have to several reactions to prepare alkyl benzene. Here, we are going to discuss how we are prepare alkyl benzene step by step.
Preparing phthalic acid and terephthalic acid from benzene
There are common reactions for preparing phthalic acid and terephthalic acid preparing from benzene.
Friedel craft alkylation - methyl benzene preparing
Benzene is treated with methyl chloride in the presence of anhydrous AlCl3. It gives methylbenzene as the product.
1,2-Dimethylbenzene preration - Methyl benzene and methyl chloride in the presence of anhydrous AlCl3
Again, with excess methyl chloride in the presence of anhydrous AlCl3, methyl benzene give two products. They are 1,2-Dimethylbenzene and 1,4-Dimethylbenzene.
Separating 1,2-Dimethylbenzene and 1,4-Dimethylbenzene
We can use a chemical separation method to separate 1,2-Dimethylbenzene and 1,4-Dimethylbenzene. Distillation can be used as a separation method because boiling points of 1,2-Dimethylbenzene and 1,4-Dimethylbenzene are different.
1,2-Dimethylbenzene oxidation
1,2-Dimethylbenzene is oxidized by acidic potassium permanganate to produce phthalic acid.
1,4-Dimethylbenzene oxidation
1,4-Dimethylbenzene is oxidized by acidic potassium permanganate to produce terephthalic acid.