Calculate pH and H3O+ concentration of Weak Acid
A weak acid partially dissociate to it's ions in the water. Therefore their acidic strength of weak acid is not strong like strong acids. Weak acid dissociation is a reversible reaction. Le Chatelier's principle and equilibrium formula is applied to calculate the concentrations of each components in the solution and pH of the aqueous solution.
In this tutorial, we will discuss followings.
- Definition of Weak acid in chemistry
- Dissociation of weak acid
- H3O+ concentration and pH value
- Weak acids examples
- Examples problems for calculating H3O+ concentration, pH and weak acid
concentration
- How to calculate H3O+ concentration
- How to calculate pH of a weak acid after finding H3O+ concentration
- Calculating H3O+ concentration and pH of 1 mol dm-3 CH3COOH acidic solution
Definition of Weak acid in chemistry
When weak acid is partially dissociated to H3O+ ion and respective anion and formed anion is not stable in the water, that acid is called as a weak acid. That anion has the capability of reacting with a H2O molecule and obtain a proton from H2O molecule. Nitrous acid, acetic acid, formic acid are several examples for weak acids and we will teach you how to calculate pH of these weak acidic solutions in series of tutorials.
Dissociation of weak acid in water
As previously mentioned, a weak acid dissociates partially to hydronium ions (H3O+) ion and respective anion in water. As an example, when acetic acid (CH3COOH) is partially dissociated in water, acetate ion (CH3COO-) and hydronium ion (H3O+) ions are given.
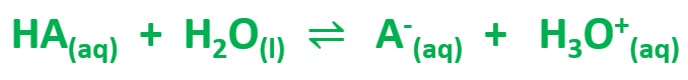
H3O+ concentration and pH value
H3O+ concentration is very low when it compares with undissociated weak acid concentration. As an example, if weak acid concentration is 0.1 mol dm-3, H3O+ concentration may be 0.0001 mol dm-3. pH of that aqueous solution is 4.
If there are two separate aqueous solutions, one is a weak acid and other one is a strong acid and both of their concentration are same, pH of weak acid is higher than the strong acid.
Weak acids examples
- Formic acid (HCOOH)
- Ethanoic acid (CH3COOH)
- Sulfurous acid (H2CO3)
- Carbonic acid (H2CO3)
- Nitrous acid (HNO2)
Examples problems for calculating H3O+ concentration, pH and weak acid concentration
Here in this tutorial, we solve few problems to calculate H3O+ ion concentration and pH of several weak acids.
How to calculate H3O+ concentration
When weak acid gained the equilibrium after some time, we can apply the equilibrium expression. At equilibrium, concentrations of weak acid, respective anion and hydronium ions are kept unchanged.
Equilibrium expression
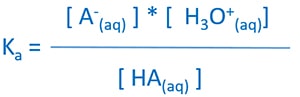
- Ka = Equilibrium Constant
- [HA(aq)] = Equilibrium concentration of acid
- [A-(aq)] = Equilibrium concentration of anion
- [H3O+(aq)] = Equilibrium concentration of hydronium ion
Ka depends on the acid and the temperature. When concentrations are taken in mol dm-3 units, Ka also has the mol dm-3 units.
Ka value is different for CH3COOH acid and HCOOH acid. Also Ka is different for 200C and 300C for HCOOH acid.
When Ka and weak acid concentration are given, H3O+ concentration and pH can be found easily by making some simple assumptions. These assumptions are explained in following examples.
How to calculate pH of a weak acid after finding H3O+ concentration
Substitute calculated H3O+ concentration to the pH equation.
pH = -log10[H3O+(aq)]
Example calculation
Calculating H3O+ concentration and pH of 1 mol dm-3 CH3COOH acidic solution.
There is a CH3COOH acid solution in the laboratory. Initial CH3COOH(aq) concentration is 1 mol dm-3. After obtaining the equilibrium states, calculate followings and mention all the assumptions you made in calculations.
- Equilibrium [H3O+(aq)] concentration
- pH of the solution
Ka of CH3COOH(aq) = 1.8 * 10-5 mol dm-3
Solution
First look what are the data given for you. Only Ka of CH3COOH and initial CH3COOH(aq) concentration are given to solve the question.
Assumptions made in the calculations
- Dissociation of water is negligible when it comparing with weak acid dissociation. Therefore we can assume, H3O+(aq) is given by weak acid dissociation. And also pH of the solution totally depends on the weak acid.
- After gaining the equilibrium, weak acid concentration is same as the initial weak acid concentration because dissociated amount (concentration) is very small comparing with initial amount (initial concentration).
Chemical reaction between CH3COOH and water
Write the chemical reaction between CH3COOH and water. Then construct a table as below and write down the three rows as initial, dissociated / formed, and equilibrium concentrations with mol dm-3.
We take dissociated concentration as a mol dm-3. When a amount concentration is dissociated, a amount concentrations of CH3COO- and H3O+ are formed.
Then we know the equilibrium concentrations. We neglect water concentration because water concentrations is very larger than other components.
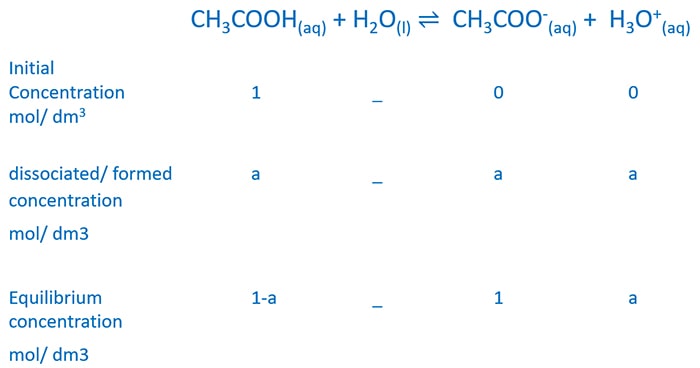
Substitute equilibrium concentrations to equilibrium expression
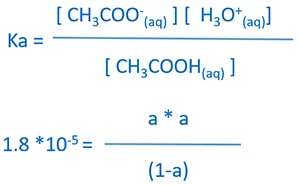
According to the assumptions we made earlier, we can assume equilibrium CH3COOH concentration equals to the initial CH3COOH concentration. Therefore equilibrium CH3COOH concentration = 1.0 mol dm -3.
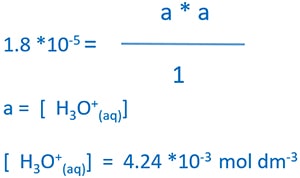
Calculate pH of CH3COOH solution
Substitute calculated H3O+ concentration to the pH equation.

Have Questions?
Related tutorials to weak acids