Aniline to benzene organic conversion
Aniline to benzene organic conversion has two steps. First, benzenediazonium chloride should be prepared by aniline. Then benzene can be prepared by benzenediazonium chloride .
Methods of convert aniline to benzene
There are two methods to prepare benzene from aniline.
- Aniline to benzenediazonium, benzenediazonium to benzene
- Aniline to phenol, phenol to benzene
Benzene to aniline | preparation of aniline from benzene
Preparing benzene from aniline through benzenediazonium
Prepare benzenediazonium salt from aniline
Prepare benzenediazonium chloride from aniline using nitrous acid ( NaNO2 / HCl ) . To benzenediazonium chloride is prepared, temperature should be kept under 50C.
Benzenediazonium salt to benzene
Then add H3PO2 or CH3CH2OH to benzenediazonium chloride to prepare benzene.

Physical properties of aniline, benzene
There are differences in properties of aniline and benzene. That can used to identify aniline and benzene.
- Both aniline, benzene are liquids in room temperature.
- Aniline is brown colour, benzene is colourless.
- Aniline has basic characteristic, benzene does not have.
- Aniline can make hydrogen bonds.
- Aniline has higher boiling point than benzene.
How to identify aniline and benzene
Aniline reacts with bromine liquid and give white precipitate, 2,3,4-tribromoaniline. But benzene does not react with liquid bromine.
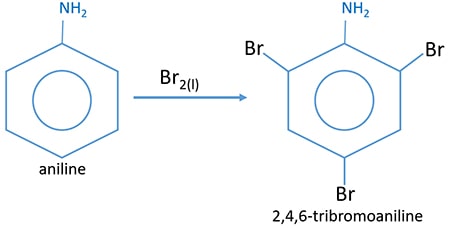
Aniline is a primary amine which reacts with dilute acids such as HCl, H2SO4 and give salts. This salt is soluble in water.
But benzene does not react with dilute acids.
How to convert aniline to benzene through phenol
Aniline to benzene through phenol has only two steps. First, aniline reacts with nitrous acid in room temperature. It gives phenol. Then phenol is distilled with zinc powder to give benzene.
Nitrous acid - HNO2
Conversion of aniline to benzene in school chemistry examinations.
Aniline to benzene is a famous conversion.