C2O42- (Oxalate ion) Resonance Structures
Resonance structures of oxalate ion (C2O42-) ion can be drawn by using lewis structure of oxalate ion. four stable resonance structures can be drawn for C2O42- resonance structures. These structures are used for to construct resonance hybrid of C2O42-.
Lewis structure of oxalate ion (C2O42-)
Lewis structure of C2O42- ion is important because it is required to draw resonance structures of oxalate ion. This means, first you have to draw the lewis structure of C2O42- before drawing the resonance structures.
Resonance structures of C2O42- ion
Let's draw four stable four resonance structures for the C2O42- ion. When you draw, resonance structures, locations of atoms should not be changed. You should only change lone pairs on atoms and bonds between atoms. Study how lone pairs and bonds are changed in following resonance structures of C2O42- ion.

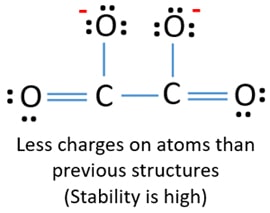
Lone pairs, charges and bonds of C2O42- ion
You may see, overall charge of all structures are -2.
Oxygen atoms love to keep negative charges than carbon atoms does because electronegativity of oxygen atom is greater than carbon atoms.
When we draw resonance structures, we should convert lone pairs to bonds and bonds to lone pairs when it is possible. But, when we do this, you should be careful to protect stability of ion and octal rule (only applicable for 1st and 2nd periods).
In lewis structure of C2O42- ion, there are three lone pairs (in the last shell) in two oxygen atoms. Also, those oxygen atoms have a -1 charge on each atoms.
There are two another oxygen atoms. Those two oxygen atoms are connected to the carbon atoms by a double bond has two lone pairs in its last shell. Also, there are no charges in those oxygen atom.
On carbon atoms, there are no lone pairs or charges.
Questions asked by students
Ask your question and find the answer free.