ClO4- (Perchlorate ion) Resonance Structures
Resonance structures of ClO4- ion can be drawn by using lewis structure of perchlorate ion. four resonance structures can be drawn for ClO4-resonance structures. Stable resonance structure, are used to draw resonance hybrid.
Lewis structure of perchlorate ion (ClO4-)
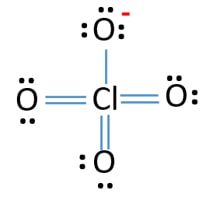
Lewis structure of ClO4-4-
Resonance structures of ClO4- ion
Let's draw four stable four resonance structures for the phosphate anion (NO3-). If you are asked to draw resonance structures of ClO4-, you should draw them first, because they are the most stable ones.

Lone pairs, charges and bonds of ClO4- ion lewis structure
- When we draw resonance structures, we convert lone pairs to bonds and bonds to lone pairs when it is possible. When we do this, you should be careful to protect stability of ion, octal rule and more.
- In lewis structure of ClO4- ion, there are three lone pairs (in the last shell) in one oxygen atom and that oxygen atoms have a -1 charge on each atoms.
- There are another three oxygen atoms. those oxygen atoms are connected to the chlorine atom from double bonds and have two lone pairs in their last shell. Also, there is no charge in those oxygen atoms.
- On chlorine atom, there are no lone pair or a charge.
Steps to draw resonance structures for N2O4
You should follow these guidelines in the drawing resonance structures for any molecule or ion.
- Structure (positions) of lewis ion should not be changed.
- Overall charge should not be changed though charge of atoms are changed.
- Following octal rule is a must for first two period atoms.
You can convert a lone pair of one oxygen atom which already has three lone pairs to make a bond with chlorine atom. (In ClO4- lewis structure, there is only one oxygen atom which has three lone pairs) With that, total electrons around chlorine atom is going to be sixteen. It is not a problem because chlorine can keep more than eight electrons in its valence shell (chlorine has 3d orbitals which help to keep more than eight electrons in its last shell. Chlorine can keep 18 electrons in its last shell if it is required).

With that electron transferring, number of bonds around the chlorine atom become 8 and chlorine atom gains -1 charge. Therefore, this is not a best resonance structure because oxygen should hold the negative charges (electronegativity of oxygen is higher than phosphorous). Otherwise, we can say oxygen has the great potential to keep negative charges than chlorine atom.
To obtain a stable resonance structure, convert a bond of former double bond to a lone pair as in the figure.
Now, chlorine atom does not have a charge and only one oxygen atom has a charge. As like this, we can convert lone pairs to bonds and vice versa to draw four resonance structures of chlorate ion.
You can see, -1 charge is moved from one oxygen atom to another oxygen atom. Also, a single bond became a double bond and double bond became a single bond.
Questions asked by students
Ask your question and find the answer free.