Lewis Structure of Oxygen Difluoride (F2O)
Oxygen difluoride (F2O) has two fluorine atoms and one oxygen atom. In the lewis structure of F2O, two fluorine atoms are joint with center oxygen atom. Also, there are no charges on atoms in F2O molecule. In this tutorial, you will learn how to draw the lewis structure of oxygen difluoride. So, you will learn how to properly draw a lewis structure of a molecule .
F2O lewis structure
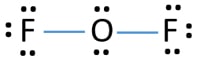
Steps of drawing lewis structure of F2O
Following steps are the general guidelines to draw a lewis structure. Those rules are applied to draw the F2O lewis structure and they are explained in detail in next sections of this tutorial. If you are are beginner to lewis structure drawing, follow these sections slowly and properly to understand.
- Find total number of electrons of the valance shells of fluorine and oxygen atoms
- Total electrons pairs
- Center atom selection
- Put lone pairs on atoms
- Check the stability and minimize charges on atoms by converting lone pairs to bonds until most stable structure is obtained.
Total number of electrons of the valance shells of F2O
- Fluorine is a group VIIA element. Therefore, fluorine has seven electrons in its last shell.
- Oxygen is a group VIA element. Therefore, oxygen has six electrons in its last shell.
- Total valence electrons given by oxygen atoms = 6 * 1 = 6
There are two fluorine atoms in F2O molecule, Therefore
- Total valence electrons given by fluorine atom = 7 * 2 = 14
- Total valence electrons = 14 + 6 = 20
Total valence electrons pairs
Total valance electrons pairs = σ bonds + π bonds + lone pairs at valence shells
Total electron pairs are determined by dividing the number total valence electrons by two. For, F2O, total pairs of electrons are 10 (=20/2).
Center atom and sketch of F2O molecule
There are some requirements to be the center atom. Having a high valence is a leading requirement to be the center atom. In oxygen difluoride, there are only two elements to select the center atom.
- Fluorine's maximum valence is 1. Oxygen's maximum valence is 2
- Oxygen is more electropositive than fluorine (fluorine is the most electronegative element in the periodic table).
- From above two facts, we can decide oxygen should be the center atom of F2O.
Mark lone pairs on atoms
- There are already two F-O bonds in the above sketch. Therefore only eight (10-2 = 8) valence electrons pairs are remaining to draw the rest of the structure.
- Start to mark remaining eight valence electrons pairs as lone pairs on outside atoms (on fluorine atoms). You can mark three lone pairs on one fluorine atom. As like that, two fluorine atoms will take six lone pairs.
- Then, there are two (8-6) many electron pairs to mark on oxygen atom.

Charges on atoms
There are no charges on atoms. Therefore, this structure should be the lewis structure of F2O.
Questions