NO2- Resonance Structures (Nitrite ion)
When we drew lewis structure of NO2- ion, we can draw resonance structures of NO2- ion. In this tutorial, you can see how many resonance structures can be drawn for nitrite ion (NO2-) and what are the steps you need to know. Also, we will discuss which resonance structures are more stable an which are less stable.
Lewis structure of nitrite ion (NO2-)
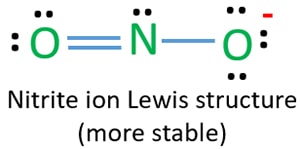
Lewis structure of NO2- ion is important because it is required to draw resonance structures.
Resonance structures of NO2-
Lets draw the two resonance structures for the nitrite anion NO2-
Lone pairs, charges and bonds of NO2- ion
When we draw resonance structures, we convert lone pairs to bonds and bonds to lone pairs if it is possible.
In lewis structure NO2- ion, there are three lone pairs (in the last shell) in one oxygen atom and that oxygen atom is joint with nitrogen atom by a single bond. Also, that oxygen atom has a -1 charge.
There is another oxygen atom. That oxygen atom is connected to the nitrogen atom by a double bond has two lone pairs in its last shell. Also, there is no charge in that oxygen atom.
On nitrogen atom, there is only a single lone pair. There is no charge on nitrogen atom too.

Steps to draw resonance structures for NO2-
You can convert a lone pair of one oxygen atom which already has three lone pairs to make a bond with nitrogen atom. With that, total electrons around nitrogen atom is going to be ten. It is going to break octal rule because nitrogen atom cannot keep more than eight electrons in its last shell. Then, what will do?
Now, we convert a bond (in the double bond) between nitrogen atom and other oxygen atom to a lone pair on oxygen atom.
Negative charge of oxygen atom is now shifted to other oxygen atom.
Now, we have a resonance structure of NO2-.

How many resonance structures are possible for NO2- ion?
Only two resonance structures are possible for NO2- ion.
Questions asked by students. Ask your question and find the answer free.
draw the lewis structure of the nitrite ion. how many resonance structures must be drawn?
After drawing the lewis structure, try to draw the resonance structures which are stable. Resonance hybrid is drawn by stable resonance structures. Therefore, many resonance structures must be drawn is depend on the number of stable resonance structures. For nitrite ion, it is two.
how many resonance structures does nitrite have?
We can draw two stable resonance structures for nitrite ion. If want, can draw unstable structures too.
draw all resonance structures for the nitrite ion , no2-
If question is asked like this, you have to draw all resonance structures possible to draw. You should include unstable one's too. In this tutorial, we have shown you what are the stable resonance structures of NO2- ion.
Is NO2- resonance structures and NO2 resonance structures are different?
Yes. They are different because total valance electrons of two molecules are different. Hence lewis structures of two molecules are different and their resonance structures are also different.
Is NO2 a resonance structure of NO2-
No. Check the lewis structure of NO2 and resonance structures of NO2.
no2 ion
There is no NO2 ion. NO2 is a brown color gaseous molecule.