NO3- Resonance Structures (Nitrate ion)
When we have finished the drawing of lewis structure of NO3- (nitrate) ion, we can draw resonance structures of NO3- ion. You can see how many resonance structures can be drawn for nitrate ion (NO3-).
Lewis structure of nitrate ion (NO3-)
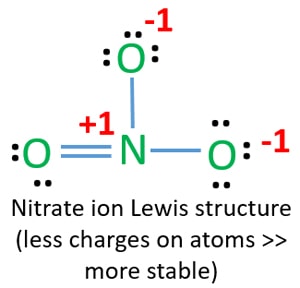
Lewis structure of NO3- ion is important because it is required to draw resonance structures of nitrate.
Resonance structures of NO3- ion
Lets draw the three resonance structures for the nitrate anion (NO3-)
Lone pairs, charges and bonds of NO3- ion
When we draw resonance structures, we convert lone pairs to bonds and bonds to lone pairs when it is possible.
In lewis structure of NO3- ion, there are three lone pairs (in the last shell) in two oxygen atom and that oxygen atoms. Also, those two oxygen atoms has a -1 charge.
There is another oxygen atom. That oxygen atom is connected to the nitrogen atom by a double bond has two lone pairs in its last shell. Also, there is no charge in that oxygen atom.
On nitrogen atom, there are no lone pair. But there is +1 charge on nitrogen atom.
Steps to draw resonance structures for NO3-
You can convert a lone pair of one oxygen atom which already has three lone pairs to make a bond with nitrogen atom. With that, total electrons around nitrogen atom is going to be ten. It is going to break octal rule because nitrogen atom cannot keep more than eight electrons in its last shell. Then, what will do?
Now, we convert a bond (in the double bond) between nitrogen atom and other oxygen atom to a lone pair on oxygen atom.
Negative charge of oxygen atom is now shifted to other oxygen atom.
Now, we have a resonance structure of NO3-.

How many resonance structures are possible for NO3- ion?
Three stable resonance structures are possible for NO3- ion. If you prefer, you can draw unstable resonance structures.
Stable resonance structures of nitrate ion

Questions asked by students
Ask your question and find the answer free.In the Nitrate resonance drawings, do the three structures exist simultaneously or do the rotate from one to another to the third?
There is no three resonance structures existing simultaneously and those resonance structures are not rotating from one structure to another. What actually happening is, there is only one real structure for nitrate ion (resonance hybrid). To draw that, we use above three resonance structures.
in the lewis structure for nitrate ion, NO3-, what is the total number of reasonable resonance structures that can be drawn?
Three reasonable resonance structures can be drawn. Each of those resonance structures, two oxygen atoms have charges (each one has -1) and nitrogen atom has a +1 charge. Therefore all of them can be considered they have same stability.
each of the three resonance structures of no3– has how many lone pairs of electrons?
There are eight lone pairs of electrons in each of the three resonance structures of NO3- ion. These eight lone pairs are provides by three oxygen atoms. There are no lone pairs on nitrogen atom.
draw the lewis structures for three resonance forms of nitrate NO3-
Each of stable resonance structure of nitrate ion is a lewis structure.
does NO3- have resonance?
Yes. We can draw three resonance structures for NO3-. You can decide this from looking about whether atoms have lone pairs or double bonds. In oxygen atoms of NO3-, there are lone pairs with -1 charges. Therefore, we can draw resonance structures for nitrate ion.
How many oxygen atoms have -1 charge in resonance structures of NO3- ion. Does that number change from resonance structure to resonance structure?
In all stable resonance structures of nitraate ion, two oxygen atoms have charges.
how many resonance structures for no3-?
There are three resonance structures for NO3- ion.