Nitrogen trichloride (NCl3) Molecule Shape, Geometry, Hybridization
Lewis structure of Nitrogen trichloride (NCl3) molecule contains three sigma bonds and one lone pair around nitrogen atom. Therefore, there are total of four electrons regions around nitrogen atom which is important to decide geometry, shape and hybridization of a molecule. So, hybridization of phosphorus atom is sp3. Because there are four electrons regions, geometry is tetrahedral and shape is trigonal pyramidal.
NCl3 lewis structure
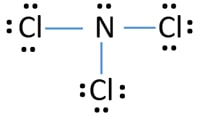
To decide the geometry, shape and hybridization of a NCl33 molecule, drawing the correct lewis structure is very important.
Number of electron regions in phosphine molecule
Total electron region is taken by the summation of sigma bonds and lone pairs around nitrogen atom.
Number of electron regions around phosphorus atom in NCl3
According to the lewis structure given above, there are three sigma bonds and one lone pair around the phosphorus atom. Therefore, total number of electron regions around nitrogen atom is four.

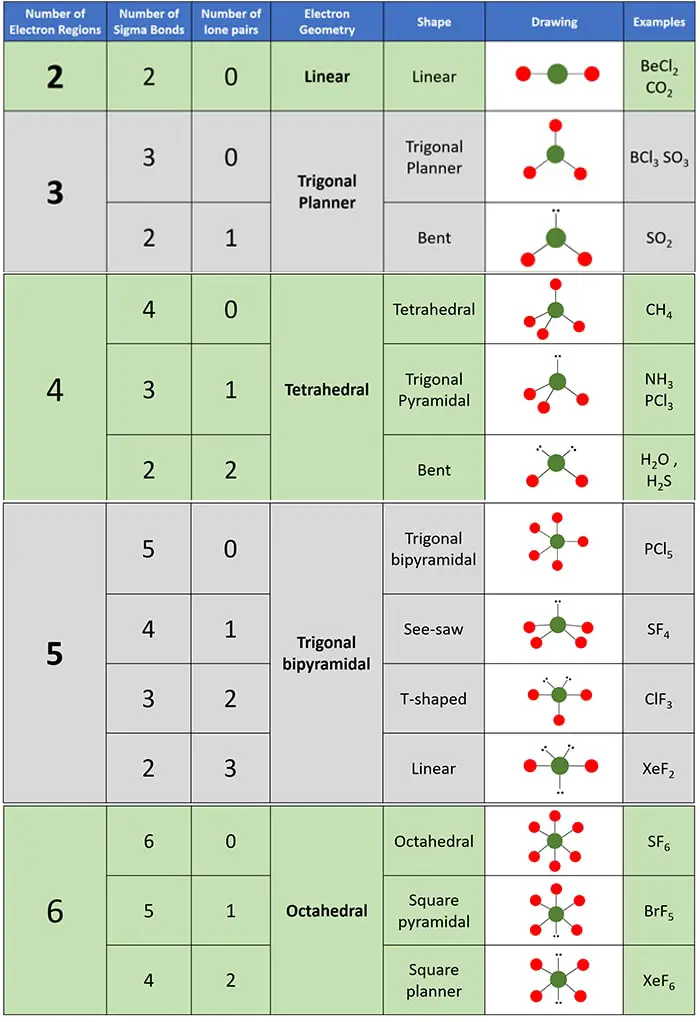
Geometry and shapes of molecules according to the number of electron regions
You can decide geometry and shapes of molecules from following table according to the number of electron regions.
- Geometry is decided by the total number of electron regions.
- Shape of molecule is decided by how many lone pairs and how many sigma bonds are located around the center atom under specific total number of electron regions.
Geometry around nitrogen atom of nitrogen trichloride
Because, number of electron regions around nitrogen atom is four according to the lewis structure, geometry around nitrogen atom should be tetrahedral.
Molecular shape of nitrogen trichloride
Because, there are three sigma bonds and one lone pair around nitrogen atom, molecular shape of phosphine is trigonal pyramidal.

Hybridization of nitrogen trichloride molecule
Hybridization is also decided by the number of electron regions around an atom. A reference table is given below for identifying hybridization.
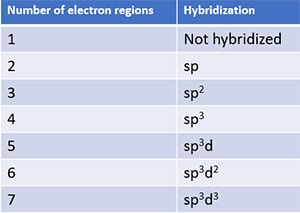
Because number of electron regions around phosphorus atom is four, hybridization of nitrogen trichloride atom is sp3
Questions
What are the similar molecules which have similar geometry, shapes and hybridization to nitrogen trichloride?
Nitrogen trichloride is very much similar to nitrogen trifloride considering both center atom and outside atoms because both outside atoms have three lone pairs in their valence shells. However, if only center atoms of molecules are considered, ammonia also has similar geometry, shape and hybridization to nitrogen trichloride molecule.