Propane (CH3CH2CH3 / C3H8) Lewis Structure
Propane (CH3CH2CH3 / C3H8) contains three carbon atoms and eight hydrogen atoms. In C3H8 lewis structure, all hydrogen atoms have made single bonds with carbon atoms. There are eight C-H bonds and two C-C bonds in C3H8 lewis structure. Also no any lone pair exist on valence shells of carbon atoms.
C3H8 lewis structure
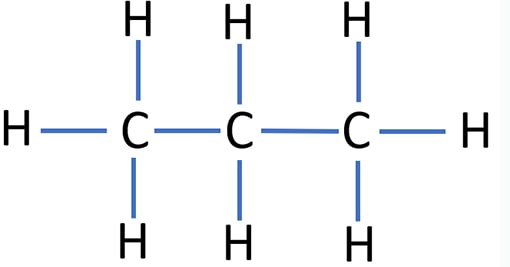
Specifications of lewis structure of Propane
- Because Propane is an alkane compound, there should be single bonds between carbon atoms.
- In the lewis structure of propane, all bonds are single bonds. All hydrogen atoms has made single bonds with carbon atoms.
- There are two C-C bond between three carbon atoms.
- There are no lone pairs on valence shells of carbon atoms.
- Also, there are no charges too in hydrogen and carbon atoms.
Center atom of C2H8 lewis structure
Because there are ten atoms (three carbon atoms and eight hydrogen atoms) in C3H8 molecule, selection of center atom may be tricky. But, because hydrogen atom cannot be a center atom in a molecule, carbon should become the center atom. Because, there are three carbon atoms, those carbon atoms should become center atoms and hydrogen atoms should be located around carbon atoms.
Questions
Can I suggest Propane is combustible by observing it's lewis structure?
Propane is an organic compound and belongs to the alkane compound type. To decide the combustibility of a reaction, it is not required to observe lewis structure of that molecule.
How many single bonds are there in Propane?
All bonds in Propane molecule are single bonds. There are two C-C bonds and eight C-H bonds. So, there are total eleven single bonds in Propane molecule.
How propene's lewis structure gets different from propane's lewis structure?
Both propane and propene has three carbon atoms. Because, propene is an alkene compound, it should contain a double bond. So, difference of propene's lewis structure is that having a double bond between two carbon atoms compared to ethane's lewis structure. Also, there are only six hydrogen atoms in propene compared to eight hydrogen atoms in propane.