Oxidation Number of Silicon, Chlorine Atoms in Silicon Tetrachloride (SiCl4)
Silicon tetrachloride, is an inorganic compound has one silicon atom and four chlorine atoms. In this tutorial, we are going to learn how to find oxidation numbers of atoms in silicon tetrachloride molecule.
There are two methods to determine oxidation numbers of atoms in molecules or ions.
- Using lewis structure
- Using algebraic equation
Find oxidation numbers of atoms of SiCl4 molecule using lewis structure
First, lewis structure of SiCl4 molecule should be drawn and drawn structure is shown below.
Mark direction of electrons attracting of bonds due to electronegativity difference of atoms
Atoms which have higher electronegativity values, can attract electrons of bonds towards them.
Electronegativity values of silicon and chlorine atoms - Pauling Scale
- Silicon: 1.90
- Chlorine: 3.16
Now, we can look how electrons are attracted between silicon and chlorine atoms.
- Among silicon and chlorine atoms Chlorine is the most electrogetive element. Due to higher electronegativity, electrons of Si-Cl bonds are attracted towards chlorine atoms as following image.
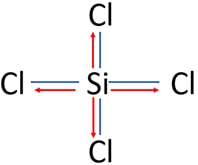
oxidation number of chlorine atoms in SiCl4
- Oxidation number of chlorine atoms: According to the lewis structure of SiCl4, electrons of the Si-Cl bond tend to attract towards chlorine atom. Therefore, silicon partially loses its electron and chlorine partially receives one more excess electron. Due to that excess electron (partially received), chlorine's oxidation number becomes -1. So, each chlorine atom's oxidation number is -1.
Oxidation number of silicon atom in SiCl4
- Oxidation number of silicon atom: Because silicon has four bonds around it, oxidation number should be calculated by taking the summation of individual oxidation numbers caused due to the bonds.
- Due to Si-Cl bonds: Because silicon partially loses an electron due to one Si-Cl bond, silicon receives +1 oxidation state.
- Because, there are four Si-Cl bonds around silicon atom, add individual oxidation numbers to find the final oxidation state.
- Oxidation state of silicon atom = (+1) + (+1) + (+1) + (+1) = +4
Find oxidation numbers of atoms of SiCl4 using algebraic equation
This method use an mathematical algebraic equation to find the oxidation number of an atom. In this method, you can find the unknown oxidation number of an atom by using known oxidation numbers of other atoms in the molecule or ion.
This method is not 100% accurate for all molecules and ions, but can be used in lot of applications.
Oxidation numbers of chlorine
Chlorine has multiple oxidation numbers such as -1, 0, +1, +3, +5, +7. Because, chlorine is more electronegative than silicon, chlorine's oxidation number should be a negative one. So, chlorine;s only negative oxidation number is -1 and we can substitute that value in our following algebraic equation.
Summation of individual oxidation numbers of each atom = overall charge of molecule or ion
For SiCl4 molecule, overall charge is zero and we can write following algebraic equation.
Oxidation number of silicon atom + oxidation number of chlorine atoms = overall charge of SiCl4 molecule
Because oxidation number of silicon atom is taken as unknown, take it as x.
Apply known values for the equation.
- x + (-1)*4 = 0
- x = +4
We have found that, oxidation number of silicon in SiCl4 molecule is +4.
Questions