Molar Balance Equation Example Problems - Reaction Engineering
In previous lesson, we have obtained molar balance equations for different reactors. Here we are going to apply that molar balance equation for a CSTR reactor to familiar with that equation.
Let's summarize some of molar balance equation parameters we learnt earlier.
General molar balance equation
In + Generation = Out + Accumulation + Loss
Generation can be positive or negative. Generation (producing species) and consumption (consuming species due to reaction) both are considered as generation in the molar balance equation. Generation is taken as positive while consumption is negative.
In our calculations, we consider losses in the reactor (system) is negligible or no losses. So we can write our new equation as following.
In + Generation = Out + Accumulation
CSTR reactor
CSTR means continuous stirred tank reactor
Problem
There is a CSTR which has inflow and outflow operates at steady state. A and B are reactants which enters to the reactor at a rate of 10.01 mol/min. A and B is react and produce C and D.
A + B → 2C + D.
at steady state, A is reacted at a rate of 8.94 mol/min, Calculate followings.
- producing rate of products
- rate of A and B in inflow
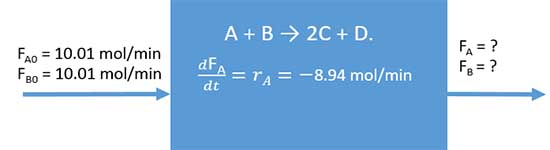
Answer
a)
According to the reaction, one A molecule and one B molecule react together and produce two C molecules and one D molecule. Therefore producing rate of C is twice as reducing rate of A.
How to find reaction rate equation of reaction and with stoichiometry.Usually reaction rate is defined as difference of amount of species per unit volume per unit time ( mol dm-3 s-1 or kmol dm-3 hr-1 ). But reaction rate of A is given as mol/min in this problem. So reaction rate of A is defined for total volume of the reactor.
- producing rate of C = 8.94 mol/min * 2 = 17.88 mol/min
- producing rate of D = 8.94 mol/min
b)
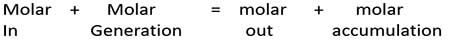
This reaction occurs in steady state. Therefore no accumulation in this reactor. Here we are going to apply our quantities in moles. Our time basis is per minute.
Apply molar balance for reactant A
A is being reacted and reducing (consumed) in the reactor. So generation is negative.
flow rate of A in inflow, FA|in = 10.01 mol/min
Generation of A, rA= -8.94 mol/min
Accumulation = 0
Substitute those values to the molar balance equation,
10.01 + ( - 8.94 ) = FA|out + 0
FA|out = 1.07 mol/min
Flow rates of A and B in inflow is same. Also A and B reacts 1:1. therefore reaction rate of A and B is same and also flow rate of B in outflow is same as flow rate of A in outflow.
FB|out = 1.07 mol/min