C4H8O Isomers | Chain, Functional, Cyclic Isomers of C4H8O
There are different isomers which can be drawn for C4H8O molecular formula. Compounds such as aldehydes, ketones, alcohols containing alkene group and cyclic alcohol compounds can be drawn for this molecular formula, C4H8O. We can identify these different isomers from several reactions which are discussed later in this tutorial.
In this tutorial, we are going to learn followings.
- Aliphatic isomers of C4H8O
- Cyclic isomers of C4H8O
- Aliphatic alcohol isomers of C4H8O which contain an alkene group
- How to identify isomers of C4H8O
First, we are going to draw aliphatic isomers of C4H8O.
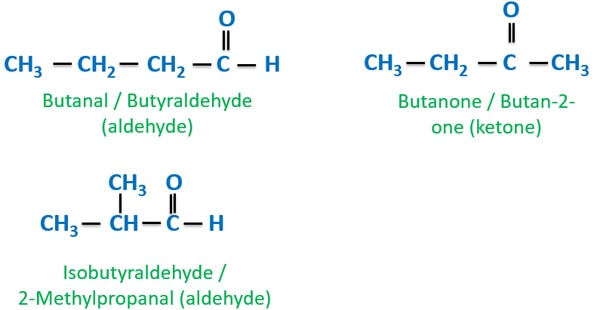
Aldehyde and ketone isomers of C4H8O
We can draw aliphatic aldehydes and aliphatic ketone for C4H8O by changing the chain of carbon atoms and functional group. So these aldehyde and ketone isomers are obtained from structural isomerism section.
Cyclic isomers of C4H8O
We can draw cyclic alcohols (not aldehydes and ketones as aliphatic compounds) for C4H8O molecular formula. Therefore, -OH group is attached with a carbon atom.

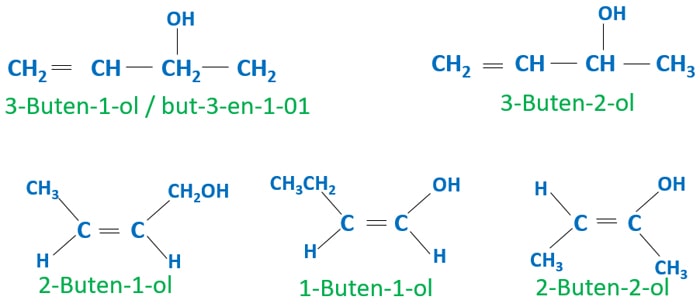
Isomers with alcohol and alkene groups
We can build some aliphatic isomers which contain alcohol and alkene groups. Some of these isomers can show optical isomerism and geometric isomerism.
How to identify C4H8O isomers?
Because, these isomers contain different functional groups (alcohols, aldehydes and ketones), they have different reactions. We use like that reactions to identify them.
Identify aldehyde and ketone compounds of C4H8O isomers
We know, aldehyde compounds can be oxidized to carboxylic acid (only formaldehyde is oxidized to carbon dioxide) by oxidizing agents such as acidic potassium permanganate. When, aldehyde is oxidized, purple colour of acidic potassium permanganate solution is reduced and become colourless or pale pink.
But, remember that ketone compounds cannot be oxidized easily as aldehyde compounds. Therefore, we can readily identify aldehyde isomers of C4H8O from ketone isomers of C4H8O.

Identify cyclic isomers of C4H8O from C4H8O aldehyde and ketone isomers
Cyclic and aliphatic isomers have different active groups. So we can identify them from reactions.
Alcohols react with sodium metal and emit hydrogen gas as product. But, aldehydes or ketones do not react with sodium. So, you can identify cyclic isomers of C4H8O from aliphatic isomers (aldehyde or ketone) of C4H8O.

Questions asked by students
Ask your question now and find the answerWhat is correct about C4H8O isomers
- There are isomers which contain carboxylic acid groups.
- There are some isomers which contain alkene group.
- There are some isomers of C4H8O, which can emit hydrogen gas with sodium
- Some isomers of C4H8O give orange colour precipitates with brady's reagent.
From above sentences, 2, 3 and 4 sentences are correct. Because there is no two oxygen atoms in C4H8O formula, there cannot exist a carboxylic group (-COOH). In some isomers, there are alkene group and hydroxyl group in same molecule.
Which functional groups contain in c4h8o isomers
When we draw all possible isomers for C4H8O, alcohol groups, alkene groups, carbonyl groups are met.
Give IUPAC names for several C4H8O isomers
There are aliphatic aldehydes, aliphatic ketones, aliphatic enols and cyclic isomers for C4H8O formula. Therefore, those isomers have different IUPAC names.
- Butanone
- Butyraldehyde
- Crotyl alcohol .
- Cyclobutano
- 1,2-Epoxybutane
- 2,3-Epoxybutane.
- Ethyl vinyl ether
- Isobutyraldehyde