Benzenediazonium Chloride Preparation, Reactions, Physical Properties
Benzenediazonium chloride is a colorless solid and have so many uses in organic chemistry. In this tutorial, we will cover how to prepare benzenediazonium chloride , its reactions and physical properties.
Chemical properties of benzene diazonium chloride
If temperature is increased in aqueous benzene diazonium chloride, it decomposes to phenol. Therefore, benzene diazonium is prepared when it is required for some purpose.
- Benzene diazonium chloride exists as a colourless solid.
- There is no melting or boiling point values because it decomposes readily.
Preparation of benzenediazonium chloride
Benzene diazonium chloride is prepared by aniline. When aniline reacts with nitrous acid under low temperature (0-50C), benzenediazonium chloride is given as the product. If temperature is increased benzene diazonium chloride decomposes to phenol. Therefore, you have to be careful with temperature when you prepare benzene diazonium chloride.
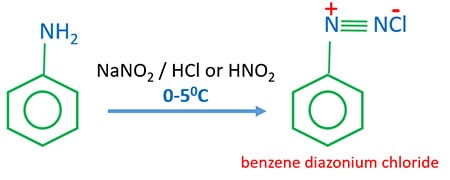
Note: In the laboratory, you will not see nitrous acid in your chemical store. Therefore you have to prepare it. Nitrous acid is prepared by mixing aqueous sodium nitrite and cold dilute hydrochloric acid.
Reactions of benzenediazonium chloride
We can categorize reactions of benzenediazonium chloride into two categories.
- Reactions of substituting diazonium group by another group
- Coupling reactions of diazonium ions
Benzene diazonium chloride is used as a raw material in the production of dyes. In next section, we will look those dye preparing reactions too.
Reactions of substituting diazonium group by another group
BenzeneDiazonium chloride (C6H5-N2Cl) can be converted to another important aromatic organic compounds such as chlorobenzene, bromobenzene, phenol and more. -N2Cl group is replaced by another group such as -Cl, -Br, -OH, etc.
Sandmeyer reactions of benzene diazonium chloride
SandMeyer reactions were found in 1884 by a Swiss chemist Traugott Sandmeyer. From sandmeyer reactions, we can prepare chloribenzene, bromebenzene, benzonitrile, iodobenzene and flurobenzene as products.
- Chlorobenzene is formed when benzenediazonium chloride is treated with CuCl (Copper(I) chloride). Instead of CuCl, you can use copper powder with HCl. In this reaction, Cu+ ion behave as a catalyst.
- Bromobenzene is formed when benzenediazonium chloride is treated with CuBr (Copper(I) bromide). Instead of CuBr, you can use copper powder with HBr. In this reaction, Cu+ ion behaves as a catalyst.
- When benzenediazonium chloride is treated with CuBr (Copper(I) bromide), Benzonitrile is produced.
- Iodobenzene can be produced by the reaction of benzenediazonium chloride and KI (potassium iodide). But, you should heat the reaction medium. For this reaction, Cu+ catalyst is not required as previous three reactions.
- You can prepare flurobenzene by the reaction of benzenediazonium chloride and HBF4 (fluoroboric acid).

Preparing benzene by benzene diazonium chloride
This is just a single step reaction. Either you use H3PO2 or ethanol as the reagents with benzene diazonium chloride.
Benzene diazonium chloride and H3PO2 reaction

Benzene diazonium chloride and ethanol reaction
Benzene, acetaldehyde and nitrogen gas are given as products by this reaction. You can see, ethanol is oxidzed to acetaldehyde which is an aldehyde compound.

Preparing nitrobenzene
When benzene diazonium chloride reacts with sodium nitrite and copper (I), nitrobenzene is given.

Coupling reactions of benzene diazonium chloride
Benzene diazonium chloride reacts with phenol, β-napthol, aniline, 2-methylaniline to form azo dyes. In these reactions, a HCl molecule is eliminated.
Phenol and benzene diazonium chloride reaction
When benzene reacts with benzene diazonium chloride, red color organic dye is prepared. This dye is used to manufacture textiles. Reaction medium should be a basic.

β-naphthol and benzene diazonium chloride reaction
This reaction gives 1-(phenylazo)-2-naphthol, orange color dye. This dye also is used to prepare color textiles. Reaction medium should be a basic.

Aniline and benzene diazonium chloride reaction
This reaction also give an organic dye. Benzene diazonium chloride reacts with aniline in basic medium to form p-aminoazobenzene which is Brownish-yellow needles with bluish cast.

N-methylaniline and benzene diazonium chloride reaction
When N-methylaniline reacts with benzene diazonium chloride in the presence of basic medium, p-methylaminoazobenzene is given.

Questions asked by students
Ask your question now and find the answerWhat is the product formed when benzenediazonium chloride is treated with cubr/hbr is?
Bromobenzene is the product formed when benzenediazonium chloride is treated with CuBr/HBr. Also, you can use Cu with HBr to prepare bromobenzene.