Propanone to Propene | Propanone (Acetone) Reduction Reactions
Propanone, the simplest ketone compound can be reduced to a secondary alcohol by a reduction reaction. There are several reducing agents which are capable of reducing ketone to secondary alcohol. Then. secondary alcohol is dehydrated to propene by a dehydrator in this organic conversion.
In this tutorial, we are going to learn followings.
- How propanone is converted to propene
- Reactions and reagents of propanone reduction
Similar tutorial: propene to propanone
Steps of propanone to propene
- Propanone is reduced to 2-propanol
- Then 2-propanol is dehydrated to propene.

Reduction of propanone to 2-propanol
Following reagents can be used as reducing agents in the reduction of ketones to secondary alcohols.
- Lithium aluminium hydride (LiAlH4)
- Sodium borohydride (NaBH4)
- Na / Ethanol
- H2 / Pt
LiAlH4 is the strong oxidizing agent. When propanone reacts with LiAlH4, 2-propanol which is a secondary alcohol is given as the product.
2-propanol dehydration to propene
2-propanol is a secondary alcohol. When secondary alcohol is heated with a dehydrator, an alkene compound is given as the product.
Dehydrators using for 2-propanol dehydration
With following dehydrators, reacting mixture should be heated. But, temperature depend on the used dehydrator.
- Concentrated H2SO4 and 1700C
- Al2O3 and 3500C
- P2O5 and heat
- H3PO4 and heat
When 2-propanol is heated with concentrated H2SO4 acid, a water molecule is eliminated to give propene.
Reduction of propanone
Already you have seen how propanone is reduced to an alcohol compound by lithium aluminium hydride or other reducing agent. Now, we are going to see, another way of propanone reduction.
Propanone reduction by clemmensen reduction
When propanone is reduced by clemmensen reduction reaction, propane is given. Propane is an alkane compound. As the clemmensen reagent, zinc amalgam and concentrated hydrochloric acid ( Zn(Hg) / conc. HCl ) is used.
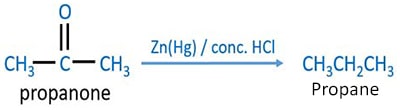
Propene to propanone
Propene can be converted to propanone by two steps.
- First, propene is hydrated by dilute H2SO4 acid to 2-propanol.
- Then, 2-propanol is oxidized by strong oxidizing agent or PCC to propanone.