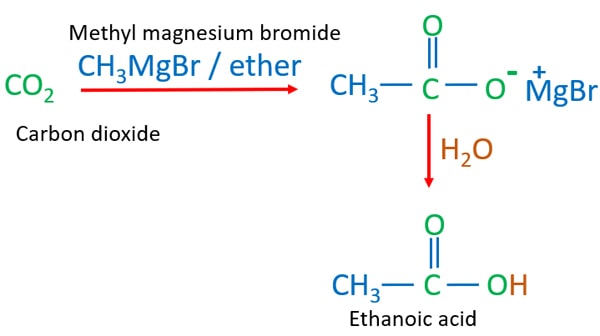
CO2 + CH3MgBr + H2O Reaction and Mechanism
When CO2 reacts with CH3MgBr (methyl magnesium bromide) and water, CH3COOH (ethanoic acid) is given as the product.
Carbon dioxide + Grignard reagent + water
Carbon dioxide reacts with a Grignard reagent compound and water and gives a carboxylic acid as the product. In this reaction, we can obtain a carboxylic acid compound increasing number of carbon atoms by one.
Carbon dioxide + Grignard reagent + water → carboxylic acid
CO2 + CH3MgBr + H2O = CH3COOH + MgBr(OH)
Carbon dioxide, methyl magnesium bromide and water reaction
The complete reaction is mentioned above. But there is a sequence to add reagents to get the desired products. Otherwise reaction will not be succeeded as purposed.
CH3MgBr exists in ether because we cannot store Grignard reagent in water or acidic medium or basic medium. Otherwise wee can say, ether is used as a solvent to keep CH3MgBr stable. CH3MgBr in ether medium exist as a solution.
How reactants are added?
- Send CO2 gas to CH3MgBr / ether solution.
- Then, add H2O to the mixture.
Mechanism of CO2, CH3MgBr and H2O reaction
In carbon dioxide molecule, carbon atom has a small positive charge because oxygen atoms are much electronegative than carbon atom.
CH3MgBr can be considered as a CH3- and +MgBr because magnesium is a highly electropositive metal. Though CH3- can behave as a nucleophile.
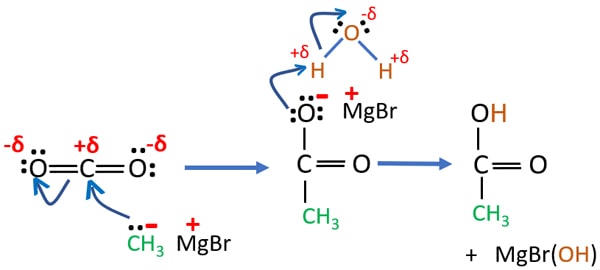
First, CH3- attacks the positively charged carbon atom. Then bonds are configured to form new C-C bond.
When water is added to the mixture, a hydrogen atom is taken by -O- and become a -OH group.
Questions asked by students. Ask your question and find the answer free.
How do I use methanol to prepare ethanoic acid using methylmagnesium bromide ?
Indirectly you are asking that how to synthesis methyl bromide (bromomethane). This is a single step reaction. Reacting methanol and PBr3 will give bromomethane (CH3Br).
What are correct statements expressed about reaction of grignard reagent with CO2
- As the product, a secondary alcohol is given.
- To get carboxylic acid as the final product, water should be added after the addition of grignard to carbon dioxide.
- From this method, formic acid can be prepared by using correct grignard reagent.
- In the presence of moisture with carbon dioxide, required product cannot be obtained.
2nd and 4th statements are correct.
Cannot use formaldehyde (HCHO) instead of CO2 to prepare ethanoic acid?
HCHO reacts with CH3MgBr and give Ethanol as the product. If CH3COOH is required, ethanol has to be oxidized by a strong oxidizing agent.
Does require excess amount of CH3MgBr to do the reaction such as some grignard reactions?
No, no need of excess CH3MgBr for this reaction because only one alkyl group is connected to the carbon dioxide molecule.
ch3cooh + ch3mgbr what will happen?
Grignard reagent will destroy and methane (CH4) is produced as a organic product.
What will happen in this reaction? ch3mgbr + h2o
Remember that, grignard reagent hydrolysis when it meets water and an alkane compound is given as the product.
Can I use acidic water instead of water to recover the ethanoic acid in final step?
Yes. Acidic water can be used. It will also increase the reaction rate.
Can I prepare ethanol from CH3MgBr + CO2?
You have to go an extra step to produce ethanol from this reaction. When carbon dioxide reacts with methylmagnesium bromide and water, ethanoic acid is given. So you have to reduce ethanoic acid to ethanol by LiAlH4.
What are the products of CH3COOH + CH3MgBr ?
Grignard reagent reacts with carboxylic acid and produce an alkane like below.
CH3COOH + CH3MgBr = CH3COO-+MgBr + CH4
CH3MgBr + CO2, what will give?
a salt is given. To recover the carboxylic acid, you have to add water as a reagent at final statge.
CH3MgBr + CO2 = CH3COO-+MgBr
CH3COO-+MgBr + H2O = CH3COOH + MgBr(OH)
Are carboxylic acids given always grignard reagent with co2?
Yes. A carbon group is joint to carbon atom in the CO2 molecule. Ethanoic acid is the simplest carboxylic acid can be prepared from this reaction. Remember that after mixing CO2 and grignard reagent , water or acidic water should be added to the reaction mixture to get carboxylic acid.
Can benzoic acid be prepared from CO2? If it possible, what are the reagent should I use?
Theoritically, it is possible. To react with CO2, phenylmagnesium bromide or phenylmagnesium chloride should be used as the grignard reagent.