FeCl3 + NaOH = Fe(OH)3 + NaCl Reaction
Aqueous ferric chloride (FeCl3) reacts with aqueous sodium hydroxide (NaOH) to produce ferric hydroxide ( Fe(OH)3 ) and sodium chloride (NaCl). So, yellow-brown color solution will turn into a brown color precipitate. Color changes, precipitating, balanced reaction, ionic equation of FeCl2 + NaOH reaction are discussed in this tutorial.
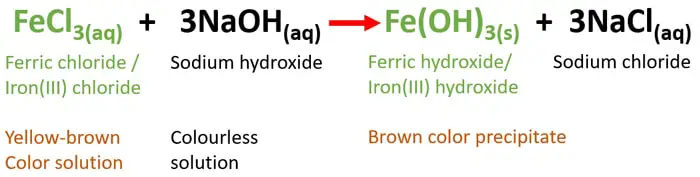
Aqueous ferric chloride and aqueous sodium hydroxide reaction
Ferric chloride ( Iron(III) chloride ) is a soluble inorganic compound in water and form a yellow-brown solution.
Sodium hydroxide is a strong alkaline colorless solution and readily gives hydroxyl ions (OH-).
What are the products when aqueous FeCl3 reacts with aqueous NaOH
When we add aqueous NaOH solution to aqueous FeCl3 solution, brown color precipitate is formed. This precipitate is ferric hydroxide ( Fe(OH)3 ).
FeCl3 + NaOH = Fe(OH)3 + NaCl
Above mentioned reaction is not a balanced chemical reaction. So, we need to balance this reaction and we will do it next section.
How to balance FeCl3 and NaOH reaction?
This reaction is a not a redox reaction. Therefore by observing number of atoms in right side and left side, we can balance the reaction in few seconds. This method is a trial and error method. But it is so easy for like this simple equation.
Balanced reaction and stoichiometry of FeCl3 and NaOH
FeCl3 + 3NaOH = Fe(OH)3 + 3NaCl
According to the stoichiometry of the reaction, 1 mol of FeCl3 reacts with 3 mol of NaOH and produce 1 mol of Fe(OH)3 and 3 mol of NaCl.
Balanced reaction of FeCl2 and NaOH with physical states
FeCl3(aq) + 3NaOH(aq) = Fe(OH)3(s) + 3NaCl(aq)
FeCl3 is soluble in water. Therefore, it exists as an aqueous solution and NaOH also exists as a solution. But, solubility of Fe(OH)3 is poor in water. Hence it deposits as a precipitate at the bottom of the reaction. NaCl is very much soluble in water and exists as an aqueous solution.
Let's solve a problem about FeCl3 + NaOH reaction
There are two aqueous solutions. In one solution, there is 0.1 mol dm-3 FeCl3 0.1 dm3 and other aqueous solution is 0.1 mol dm-3 NaOH2 0.1 dm3. When, these two solutions are mixed with each other, how much grams are precipitated as Fe(OH)3?
Answer: First, you have to find mixed amounts of NaOH and FeCl3.
- Mixed NaOH amount = 0.01 mol
- Mixed amount of FeCl2 = 0.01 mol
To react with 0.01 mol FeCl3, 0.03 mol of NaOH is required according to the stoichiometric equation. But, there is only, 0.01 mol of NaOH. Therefore, NaOH is the limited reagent.
- Produced FeCl3 amount = 0.0033 mol
- Prodyced Fe(OH)3 mass = 0.54 g
Assumption: Amount of Fe(OH)3 in the aqueous phase is negligible compared to amount in the precipitate.
Questions asked by students
Ask your question and find the answer free.
Can I prepare Fe(OH)3 from aqueous ammonia solution instead of NaOH?
If you add aqueous ammonia solution to the FeCl3 solution, you can see ferric chloride, brown color precipitate is formed.
If FeCl3 + NaOH gives Fe(OH)3 pricipitate, will it dissove in excess NaOH solution?
Fe(OH)2 is not soluble in excess NaOH or aqueous ammonia solution. So brown colour precipitate will remain in the solution.