KMnO4 + HCl | Potassium Permanaganate and Hydrochloric Acid Reaction
Aqueous hydrochloric acid (HCl) reacts with potassium permanganate (KMnO4) to produce potassium chloride (KCl), Manganese chloride (MnCl2), water (H2O) and chlorine gas (Cl2). So purplish colour solution will turn in to a colourless or light pink colour solution. Colour changes, balanced reaction, ionic equation of KMnO4 + HCl reaction are discussed in this tutorial.
Written by: Dilmi Ishanka, (undergraduate), Department of Food Science and Technology, Faculty of Agriculture, University of Peradeniya, last modified: 30/05/2021
2KMnO4(s) + 16HCl (aq) = 2KCl (aq) + 2MnCl2 (aq) + 8H2O (l) + 5Cl2(g)
Aqueous hydrochloric acid and solid potassium permanganate reaction
Potassium permanganate is available in solid form. But It is very much soluble in water. Dissolve a little bit of KMnO4 in water and then it will give a purple colour solution. When you add hydrochloric acid, you can see and emission of gas bubbles which is relevant to chlorine gas.
Colour changes when KMnO4 reacts with HCl
When reaction happens continuously purple colour of the solution is reduced with time and a colourless or light pink solution will be given when old KMnO4 is reactant.
Hydrochloric acid is a strong acid and can release H+ ions and Cl- ions to the solution. And also hydrochloric acid is a colourless solution.
What are the products when aqueous HCl reacts with solid KMnO4?
We are able to add solid KMnO4 grits to the aqueous HCl, and also we can add KMnO4 grits to the water and make a KMnO4 aqueous solution and react with HCl. When we react KMnO4 with HCl, we are able to produce chlorine gas (Cl2), potassium chloride (KCl), manganese chloride (MnCl2) and water
.Due to formation of Mn2+ ions in the solution, the solution can be seen in light pink colour. A colourless solution is occurred due to the fewer concentration of Mn2+ ions.
KMnO4 + HCl = KCl + MnCl2 + H2O + Cl2
Above mentioned reaction is not a balanced chemical reaction. So, we need to balance this reaction and we will do it in next section.
How to balance KMnO4 and HCl reaction?
This reaction is a redox reaction.
In this reaction, the oxidizing agent is KMnO4. And the reducing agent is HCl. In KMnO4, MnO4- is the oxidizing anion. In HCl, the reducing agent is Cl-.
In this KMnO4 compound, the cataion is K+ and the anion is MnO4- . Oxidation number of the K+ ion is +1. So, the oxidation number of the anion is -1.
What is the oxidation number of the Mn ion in MnO4-?
MnO4- ion is reduced in to Mn2+ ion.
Let's calculate the oxidation number of the Mn ion in MnO4-. Let's assume as the oxidation number of the Mn is x, Most times, oxidation number of the oxygen is -2. We use algebraic method to calculate the oxidation number of manganese.
- x + (-2) * 4 = -1
- x + (-8) = -1
- x = -1 + 8
- x = 7
So, oxidation number of the Mn in MnO4-- is +7.
What is the oxidation number of the Cl2 gas?
Chloride (Cl-) anaion is oxidized in to chlorine gas (Cl2). The charge of chlorine gas is 0. So the oxidation number of the chlorine atom in chlorine gas is 0.
How to balance the ionic reaction for KMnO4 + HCl?
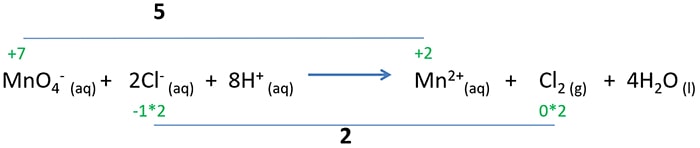
The difference of the oxidation of Cl- to Cl2 is +2. And the difference of reducing MnO4-- to Mn2+ is +5.
Now change the differences.

This is the balanced ionic reaction.
Balanced equation for KMnO4 + HCl reaction

Safety during the KMnO4 and HCl reaction
Because HCl acid is a strong acid, it can cause injuries to skin and more. Therefore be careful when HCl solution is poured into the solution of KMnO4. Also, be careful when aqueous HCl is prepared becaue HCl vapor can harl your lungs.
Also chlorine gas is a very toxic gas and inhaling chlorine gas can cause severe injuries. If you feel a chlorine gas odor, get away. This reaction should be done in a closed container to prevent release of chlorine gas to atmosphere.
How to do this HCl and KMnO4 reaction in laboratory?
Because HCl and KMnO4 are very common chemicals in a lab, you can easily find them. But, use a closed beaker to do this reaction because chlorine is a toxic gas or there should be proper removal method or collection method for chlorine gas.