Ca + HNO3 | Calcium and Nitric Acid Reaction
Calcium's reaction with nitric acid varies according to the concentration of nitric acid because nitric acid can behave as an oxidizing acid and as well as a typical acid. Therefore, products of the reaction may be different and we are going to discuss those things in detail in this tutorial.
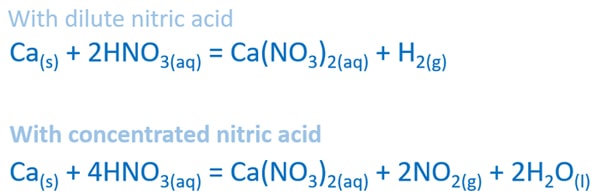
Calcium and dilute nitric acid reaction
Ca(s) + HNO3(aq) = Ca(NO3)2(aq) + H2(g)
When concentration of nitric acid is too low, it shows typical acid solution properties. Therefore, as products calcium nitrate and hydrogen gas are given.
Balanced equation of calcium and dilute nitric acid reaction
Ca(s) + 2HNO3(aq) = Ca(NO3)2(aq) + H2(g)
Calcium and concentrated nitric acid reaction
When nitric acid concentration is considerably high, it behaves as an oxidizing acid. Then, as products calcium nitrate, nitrogen dioxide and water are given as products.
Ca(s) + HNO3(aq) = Ca(NO3)2(aq) + NO2(g) + H2O(l)
You can see brown colour nitrogen dioxide gas is released when reaction is being happened.
Balanced equation of calcium and concentrated nitric acid reaction
Ca(s) + 4HNO3(aq) = Ca(NO3)2(aq) + 2NO2(g) + 2H2O(l)
How do I know what products are given?
If you can see a brown colour gas emission in the reaction, it is related to nitrogen dioxide gas. Therefore, nitric acid concentration should be high in this reaction.
In the dilute nitric acid case, hydrogen gas is released. You can check the presence of hydrogen gas by holding a burning splint near to the top of the test tube. The positive result is a squeaky pop sound due to combustion of hydrogen gas.
Questions asked by students
Ask your question and find the answer free.