Calcium carbide and Water Reaction | CaC2 + H2O
Calcium carbide (CaC2) hydrolysis and react in the water to give acetylene (C2H2) gas and calcium hydroxide. This reaction is used to prepare acetylene gas in the laboratory. As well, a white precipitated, Ca(OH)2 is formed in this reaction.
Written by: Heshan Nipuna, Eng., Chemical & Process Engineering, University of Peradeniya, last modified: 26/09/2021
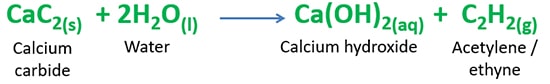
Balanced reaction of calcium carbide and water reaction is given below.
CaC2(s) + 2H2O(l) = C2H2(g) + Ca(OH)2(aq)
Physical observations during the reaction of calcium carbide and water
Calcium carbide can be seen as white powder to grey /black crystals. When you add calcium carbide crystals to water, acetylene gas is emitted. Acetylene is a colourless gas and insoluble in water.
Calcium hydroxide is also formed as a product and it exist as a aqueous colourless solution. If concentration of calcium hydroxide is high, calcium hydroxide can be precipitated as a white precipitate.
Safety during the reaction
You should be very careful to not expose a open flames or any high temperatures to emitting acetylene gas because acetylene is a highly flammable gas.
Uses of CaC2 and H2O reaction
Acetylene gas is used for ripping fruits. However, this is illegal in some countries because several toxic compounds such as phosphine and arsine are generated. But, these toxic gases can be removed by passing acetylene gas through copper sulfate solution.
Reaction of chlorine and aqueous sodium hydroxide is another example for disproportionation reaction.