CrCl3 + NaOH = Cr(OH)3 + NaCl | Chromium Chloride + Sodium Hydroxide Reaction
Chromium chloride (CrCl3) reacts with NaOH and gives chromium hydroxide (Cr(OH)3) and sodium chloride (NaCl). Chromium hydroxide is a green colour precipitate. In the presence of excess NaOH, Cr(OH)3 is dissolved and give a green colour solution. Colour changes, precipitation and more characteristics of chromium chloride and sodium hydroxide reaction are explained in this tutorial.
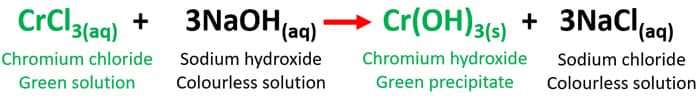
CrCl3(aq) + NaOH(aq) = Cr(OH)3(s) + NaCl(aq)
Chromium chloride is soluble in water and dissociates to Cr3+ and Cl- ions. As well as, NaCl readily dissociates to Na+ and OH- ions in water.
Aqueous chromium chloride is a green colour solution and aqueous sodium hydroxide is a colourless solution. When they react with each other, green colour precipitate, chromium hydroxide and sodium chloride is given as products.
Adding NaOH crystals to CrCl3 solution
You can see the exact results as mixing aqueous NaOH and aqueous CrCl3 when NaOH crystals are added to aqueous CrCl3.
CrCl3(aq) + NaOH(s) = Cr(OH)3(s) + NaCl(aq)
Balanced equation of chromium chloride and sodium hydroxide reaction
CrCl3(aq) + 3NaOH(aq) = Cr(OH)3(s) + 3NaCl(aq)
1 mol of CrCl3 reacts with 3 mol of NaOH and produce 1 mol of Cr(OH)3 and 3 mol of NaCl. Except Cr(OH)3, all other compounds exist as aqueous state in the equation. Cr(OH)2 exists in solid state. Remember that, to write physical states of compounds when you write the balanced equation.
Observations during the CrCl3 and NaOH reaction
When you are slowly adding one chemical to other chemical drop by drop, at one time, you will see a green colour precipitate is formed in the solution. If you suddenly add one chemical to another, green precipitate is also formed immediately and will be unavailable to detect precipitating moment.
In the presence of excess NaOH, what will happen to the green colour Cr(OH)3 precipitate?
Chromium hydroxide is soluble in excess sodium hydroxide because chromium hydroxide is an amphoteric hydroxide. Therefore, chromium hydroxide precipitate will dissolve in excess NaOH and give tetrahydroxochromate(III) ion, a coordination complex anion.
Cr(OH)3(aq) + NaOH(aq) = [Cr(OH)4]-(aq) + Na+(aq)
Questions asked by students
Ask your question and find the answer free.
What are the other 3d metal cations which will give green precipitates with aqueous NaOH?
Nickel chloride can give a green colour Ni(OH)2 precipitate with NaOH. However, Ni(OH)2 is not soluble in the presence of excess NaOH such as Cr(OH)3. Therefore, we can distinguish Ni(OH)2 and Cr(OH)3 from excess NaOH.
I want to know whether can I separate CrCl3 and FeCl2 solutions from NaOH?
Yes. You can see the precipitate given related to the CrCl3 solution will dissolve in excess NaOH. Fe(OH)2 is not soluble in aqueous NaOH.