Magnesium chloride and Sodium hydroxide Reaction | MgCl2 + NaOH
When Magnesium chloride (MgCl2) and sodium hydroxide (NaOH) solutions are mixed, a white precipitate, magnesium hydroxide (Mg(OH)2) and sodium chloride (NaCl) are given as products. Sodium chloride exists in the aqueous solution while magnesium hydroxide is precipitated in the bottom of the solution after sometime. However, small amount of magnesium and hydroxide ions exist in the aqueous solution.
MgCl2(aq) + NaOH(aq) = Mg(OH)2(s) + NaCl(aq)
Both magnesium nitrate and sodium hydroxide solutions are colourless solutions. When they react with each other, a white precipitate (Mg(OH)2) and a colourless solution is given. Sodium chloride exists in the colourless aqueous solution.
Observations during the reaction
When you are slowly adding one chemical to other chemical drop by drop, at one time, you will see a white precipitate is formed in the solution. If you immediately add one chemical to other chemical, precipitate is formed immediately and cannot identify at which point precipitated is formed.
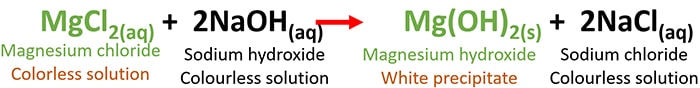
Chemically balanced equation is given above with physical characteristics such as physical states and colours.
pH change of sodium hydroxide solution
Let's assume that magnesium chloride solution added to sodium hydroxide solution.
Because sodium hydroxide is a strong base, it shows a high pH value. When sodium hydroxide react with magnesium chloride, hydroxyl ion given by sodium hydroxide is precipitated with magnesium ions. Therefore, hydroxyl ion concentration is reduced significantly and basic characteristic is also weaken. Therefore, pH value is reduced.
What will happen if solid sodium hydroxide crystals or powder form sodium hydroxide is added to aqueous magnesium chloride solution?
Sodium hydroxide readily dissociates to sodium ion and hydroxyl ion. Due to hydroxyl ion presence, immediately magnesium hydroxide precipitate will be given.
NaOH(s) → Na+(aq) + OH-(aq)
Mg2+(aq) + OH-(aq) → Mg(OH)2(aq)
Questions asked by students
Ask your question and find the answer free.