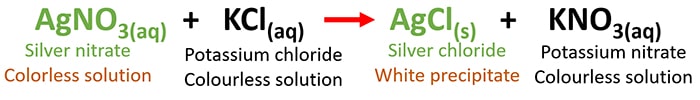
AgNO3 + KCl = AgCl + KNO3 | Silver Nitrate + Potassium Chloride Reaction
When aqueous silver nitrate (AgNO3) and aqueous potassium chloride (KCl) compounds are mixed together, there is a high chance of giving a white colour precipitate if silver nitrate and potassium chloride concentrations are considerably high. This reaction can be used to test the presence of chloride ion.
AgNO3(aq) + KCl(aq) = AgCl(s) + KNO3(aq)
Both silver nitrate and potassium chloride solutions are colourless solutions. When they react with each other, a white precipitate (AgCl) and a colourless solution is given. Potassium nitrate is a colourless water soluble compound.
Observations during the reaction
When you are slowly adding one chemical to other chemical drop by drop, at one time, you will see a white precipitate is formed in the solution. If you immediately add one chemical to other chemical, precipitate is formed immediately. But, remember that to be careful with silver nitrate during the experiment silver nitrate is a corrosive chemical.
Required concentration to see the AgCl precipitation
When same volumes of KCl and AgNO3 solutions are mixed, will there be a precipitate? Each solution has concentrations of 0.1 mol dm-3.
Let's think, when both solutions are mixed, there is no precipitate and then calculate concentration of each ion. Because each compound is diluted by two times, concentration of KCl and AgNO3 is reduced by half.
Then each compound has the concentration of 0.05 mol dm-3.
Then we can apply Ksp expression to check whether will there be a AgCl precipitate.
Initial Cl- concentration after mixing = 0.05 mol dm-3
Initial Ag+ concentration after mixing = 0.05 mol dm-3
Ksp expression for AgCl = [Ag+(aq)][Cl-(aq)]
[Ag+(aq)][Cl-(aq)] = 0.05 mol dm-3 * 0.05 mol dm-3
[Ag+(aq)][Cl-(aq)] = 2.5 * 10-3 mol2 dm-6
Ksp, AgCl = 1.7 * 10-10 mol2 dm-6
Because calculated value for Ksp expression is greater than Ksp value of AgCl. Therefore you can see AgCl is precipitated in the solution.
What will happen if solid potassium chloride is added to aqueous silver nitrate solution?
Solid potassium chloride will immediately dissociates to potassium ions and chloride ions when it is added to silver nitrate solution. Then, due to presence of silver and chloride ions, silver chloride will be precipitated as usual.
Questions asked by students
Ask your question and find the answer free.