Propanol and Oxygen Reaction (Combustion) | CH3CH2CH2OH + O2
Propanol, an alcoholic compound in organic chemistry, readily burns with oxygen gas and heat is released as a result of combustion. As chemical products, carbon dioxide and water are given if complete combustion of propanol is achieved. Otherwise, some amount of carbon monoxide can be given as another product if the supplied oxygen gas amount is not sufficient for a complete combustion.
CH3CH2CH2OH + O2 → CO2 + H2O
In this tutorial, we will discuss followings.
Propanol
There are several names used for propanol in chemistry as, 1-propanol, Propan-1-ol, Propyl alcohol, n-propanol.
Propanol contains three carbon atoms, one oxygen atom and eight hydrogen atoms. Pure praponol is evaporative and vapor can be ignited readily if spark and enough concentration of oxygen gas is found.
Stoichiometric balanced chemical reaction of propanol and oxygen gases
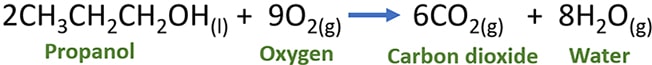
Two moles of praponol react with nine moles of oxygen gas and produce six moles of carbon dioxide and eight moles of water.
CH3CH2CH2OH(l) + 9O2(g) → 6CO2(g) + 4H2O(g)
Stoichiometric balanced equation is important how much oxygen gas is required for complete combustion of propanol.
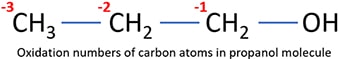
Change of oxidation numbers in propanol combustion
Because, above mentioned combustion process is a redox reaction, oxidation numbers of carbon and oxygen atoms are changed as below.
- In propanol molecule, there are three carbon atoms exist and they are in different oxidation states as following figure. However, all carbon atoms are oxidized to carbon dioxide molecules. In a carbon dioxide molecule, carbon is at +4 oxidation state. So, carbon atom is oxidized.
- Oxygen is at 0 oxidation state in oxygen molecule (O2) and that those oxygen atoms are reduced to -2 oxidation state. Therefore, oxygen in oxygen molecule is reduced during the combustion process.
Thermal energy and heat generation
Standard enthalpy of combustion of propanol (ΔHc0(CH3CH2CH2OH,(l))) = -2021.3 kJ mol-1
Health and safety
Hazards are identified as GHS classifications.
- H225: Highly Flammable liquid and vapor. If propanol vapor readily catches the fire if any spark is available.
- H336: May cause drowsiness or dizziness.
- H318: Causes serious eye damage: Serious eye damage/eye irritation
Questions