Sulfur dichloride and Water Reaction | SCl2 + H2O
Sulfur dichloride (SCl2) hydrolysis and react in water to give sulfurous acid, sulfur and hydrochloric acid. This reaction is a redox reaction and oxidation number of sulfur in sulfur dichloride is changed.
Written by: Heshan Nipuna, Eng., Chemical & Process Engineering, University of Peradeniya, last modified: 30/05/2021
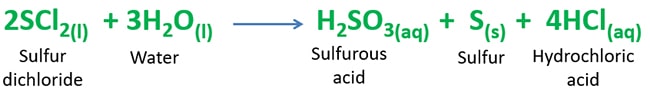
Balanced reaction of sulfur dichloride and water reaction is given below.
2SCl2(l) + 3H2O(l) = H2SO3(aq) + S(s) + 4HCl(aq)
Physical observations during the reaction of sulfur dichloride and water
Sulfur dichloride is a red colour liquid. Products given from the reaction are sulfurous acid, sulfur and hydrochloric acid.
Formation of sulfur
Because sulfur is given as a product, you can see a white-yellow precipitate is formed. Therefore, solution after the reaction is not clear.
Formation of hydrochloric acid and sulfurous acid
Usually aqueous solutions of hydrochloric acid and sulfurous acid are colourless solutions. Due to formation of these acids, pH of products' aqueous solution should be less than seven.
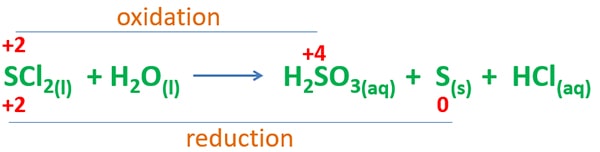
Oxidation number change in SCl2 and H2O reaction
Oxidation number of sulfur (+2) in sulfur dichloride is changed to two different oxidation states; 0 in sulfur and +4 in sulfurous acid. Therefore, this reaction is a disproportionation reaction.
Reaction of chlorine and aqueous sodium hydroxide is another example for disproportionation reaction.