Sulfur and Oxygen Gas Reaction | S + O2
Sulfur (S) is burnt with oxygen gas (O2) and gives sulfur dioxide (SO2) as the result. Sulfur exits in solid state at room temperature and sulfur dioxide exist in gaseous state. During the reaction, Sulfur is oxidized and oxygen is reduced. Sulfur and oxygen gas reaction is a redox reaction.
In this tutorial, we will discuss followings.
Stoichiometric balanced equation of sulfur and oxygen gas reaction
S(s) + O2(g) → SO2(g)
One mole of sulfur reacts with one mole of oxygen gas and one mole of sulfur dioxide are given.
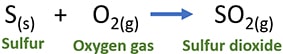
When you write the balanced equation, remember to write the physical state of chemicals.
Physical state changes
- Sulfur exists in solid state at room temperature and oxygen is a bimolecular gas
- The only product, sulfur dioxide is a gas. When sulfur dioxide is produced, heat is generated.
Health and safety measures, environmental impacts
Health and safety measures
Sulfur: Because of combustible properties, fires can be caught and finely dispersed particles can form explosive mixtures in air if any spark is caused.
Sulfur dioxide: It is a toxic gas if inhaled, possible severe skin burns and eye damage.
Environmental impacts due to combustion of sulfur
Because sulfur dioxide gas is a reason for acid rain, emission of sulfur dioxide to the environment due to the combustion of sulfur should be minimized.
Industrial applications
In sulfuric acid manufacturing process, sulfur can be used as a raw material. As the first reaction of that process, sulfur is burnt with oxygen gas to produce sulfur dioxide gas. Then sulfur dioxide is converted to sulfur trioxide (SO3) and is sent for further processes.
Questions
Can I use Sulfur as an efficient fuel in heat requirement process?
When sulfur burns, heat is released. Because Sulfur dioxide gas is generated as the product, it causes air pollution. Sulfur dioxide is a toxic gas and major chemical causing of acid rains.
Does sulfur react with oxygen?
Sulfur is a combustible solid and burns when oxygen gas is in contact.
What are the products when sulfur + oxygen gas
Generally, sulfur dioxide gas is produced as the result. Due to the combustion, energy is released.